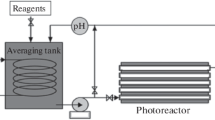
Abstract
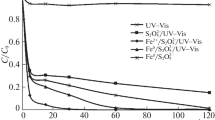
Kinetics of the oxidative destruction of para-chlorophenol in a combined iron-persulfate system under the action of simulated sunlight was studied. It was shown that, under additional photoirradiation, a deep conversion of chlorophenol and main intermediate products of its destruction is provided, with iron compounds serving not only as catalysts, but also as photochemical oxidation sensitizers. The degree of mineralization of para-chlorophenol and products of its oxidation under a photoactivated treatment for two hours reached a value of 60%, whereas that in the “dark” reaction did not exceed 1%. In the combined oxidizing system S2O 2–8 /Fe2+/UV-Vis, a considerable synergic effect was observed due to the formation of reactive oxygen intermediate both via decomposition persulfate and through reduction of Fe3+ from inactive Fe3+ intermediates.
Similar content being viewed by others
References
Kharlampovich, G.D. and Churkin, Yu.V., Fenoly (Phenols), Moscow: Khimiya, 1974.
Ahlborg, U.G. and Thunberg, T.M., Crit. Rev. Toxicol., 1980, vol. 7, pp. 1–35.
Fokin, A.V. and Kolomiets, A.F., Priroda, 1985, no. 3, pp. 3–15.
Naturally Produced Organohalogens, Grimvall, A. and de Leer, Ed. W.B., Eds., Springer Science+Business, Media, B. V., 1995.
Pera-Titus, M., García-Molina, V., Banos, M.A., et al., Appl. Catal., B, 2004, vol. 47, pp. 219–256.
Andreozzi, R., Caprio, V., Insola, A., et al., Catal. Today, 1999, vol. 53, no. 7, pp. 51–59.
Gogate, P.R. and Pandit, A.B., Adv. Environ. Res., 2004, vol. 8, nos. 3–4, pp. 553–597.
Batoeva, A.A., Ryazantsev, A.A., Sizykh, M.R., and Khandarkhaeva, M.S., Khim. Interesakh Ustoich. Razvit., 2006, vol. 14, no. 4, pp. 343–348.
Aseev, D.G., Batoeva, A.A., and Sizykh, M.R., Khim. Interesakh Ustoich. Razvit., 2009, no. 2, pp. 203–207.
Carey, J.H., Water Pollut. Res. J. Can., 1992, vol. 27, pp. 1–21.
Liu, X., Fang, L., Zhou, Y., et al., J. Environ. Sci., 2013, vol. 25, no. 8, pp. 1519–1528.
Ahmed, M.M. and Chiron, S., Water Res., 2014, vol. 48, pp. 229–236.
Chen-Ju, L. and Shun-Chin, H., Sustain. Environ. Res., 2012, vol. 22, no. 4, pp. 199–208.
Criquent, J. and Leithner, N.K.V., Chemosphere, 2009, vol. 77, pp. 194–200.
Liu, C.S., Shih, K., Sun, C.X., et al., Sci. Total Environ., 2012, vol. 416, pp. 507–512.
Hussain, I., Zhang, Y., Huang, S., et al., Chem. Eng. J., 2012, vol. 203, pp. 269–276.
Pinevich, A.V., Mikrobiologiya zheleza i margantsa (Microbiology of Iron and Manganese), St. Petersburg: S. Peterburg. Univ., 2005.
Rao, Y.F., Qu, L., Yang, H., et al., J. Hazard. Mater., 2014, vol. 268, pp. 23–32.
Fang, S.C. and Lo, S.L., Appl. Mech. Mater., 2011, vols. 121–126, pp. 2546–2556.
Weiner, E.R., Applications of Environmental Chemistry, Lewis Publishers, CRC Press LLC, Boca Raton, FL, 2000.
Legrini, O., Oliveros, E., and Braun, A.M., Chem. Rev., 1993, vol. 93, pp. 671–698.
Vorob’eva, N.I., Matafonova, G.G., and Batoev, V.B., Voda: Khim. Ekologiya, 2012, no. 9, pp. 32–36.
Batoev, V.B., Matafonova, G.G., and Filippova, N.I., Russ. J. Appl. Chem., 2011, vol. 84, no. 3, pp. 407–411).
Vichutinskaya, E.V., Pervunina, R.I., Semenova, I.V., et al., Khim. Fiz., 1997, vol. 16, no. 4, pp. 25–34.
Zhao, J., Zhang, Y., Quan, X., et al., Sep. Purif. Technol., 2010, vol. 71, pp. 302–307.
Rod’ko, I.Ya., Kozlov, Yu.N., and Purmal’, A.P., Zh. Fiz. Khim., 1999, vol. 73, no. 6, pp. 1125–1126.
Lou, X., Wu, L., Guo, Y., et al., Chemosphere, 2014, vol. 117, pp. 582–585.
Li, B., Li, L., Lin, K., et al., Ultrason. Sonochem., 2013, vol. 20, pp. 855–863.
Huang, Y.-F. and Huang, Y.-H., J. Hazard. Mater., 2009, vol. 162, pp. 1211–1216.
Kusic, H., Peternel, I., Ukic, S., et al., Chem. Eng. J., 2011, vol. 172, pp. 109–121.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.S. Khandarkhaeva, A.A. Batoeva, D.G. Aseev, M.R. Sizykh, 2015, published in Zhurnal Prikladnoi Khimii, 2015, Vol. 88, No. 10, pp. 1420-1426.
Rights and permissions
About this article
Cite this article
Khandarkhaeva, M.S., Batoeva, A.A., Aseev, D.G. et al. Photoactivation of the oxidation process of para-chlorophenol in aqueous solutions. Russ J Appl Chem 88, 1605–1611 (2015). https://doi.org/10.1134/S1070427215100080
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427215100080