Abstract
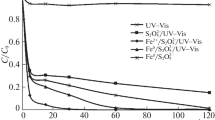
Kinetic patterns of the degradation of Methyl Orange (MO) dye are studied upon persulfate (PS) and quasi-monochromatic UV radiation (mercury-free source: KrCl-excilamp, 222 nm, referred to below as UV) treatment in the presence or absence of iron ions. Oxidative systems can be placed in the following order according to the efficiency and rate of dye degradation: {PS/UV/Fe2+} > {PS/UV} > {PS/Fe2+} > UV > PS. It is found that only in the combined {PS/UV/Fe2+} system does the total conversion of MO occur, but its deep mineralization in an aqueous solution is also possible. When this happens, the removal of total organic carbon can be as high as 77%. Inhibitors of radical reactions are used to show that both hydroxyl and sulfate anion radicals participate in oxidative degradation in the combined systems {PS/UV/Fe2+} and {PS/Fe2+}, and the role of sulfate anion radicals is dominant in the process. The possibility of using quasi-monochromatic UV radiation for persulfate activation in the oxidative degradation of azo dyes is shown experimentally.







Similar content being viewed by others
REFERENCES
N. Serpone, Y. M. Artemev, V. K. Ryabchuk, et al., Curr. Opin. Green Sustainable Chem. 6, 33 (2017).
D. B. Miklos, Ch. Remy, M. Jekel, et al., Water Res. 139, 118 (2018).
W.-L. Wang, Q.-Y. Wu, N. Huang, et al., Water Res. 141, 105 (2018).
Minamata Conventionon Mercury. http://www.mercuryconvention.org/Convention/tabid/3426/language/en-US/Default.aspx.
A. M. Boichenko, M. I. Lomaev, A. N. Panchenko, et al., Ultraviolet and Vacuum-Ultraviolet Excilamps: Physics, Technology, and Applications (STT, Tomsk, 2011) [in Russian].
M. D. Murcia, N. O. Vershinin, and N. Briantceva, Chem. Eng. J. 266, 356 (2015).
J.-W. Kang, S.-S. Kim, and D.-H. Kang, Food Res. Inter. 109, 325 (2009).
A. Aristizábal, G. Perilla, J. A. Lara-Borrero, and R. Diez, Environ. Technol. (2018). Doi https://doi.org/10.1080/09593330.2018.1494755
M. Gomez, M. D. Murcia, J. L. Gomez, et al., Chem. Eng. Process 49, 113 (2010).
G. Matafonova and V. Batoev, Chemosphere 89, 637 (2012).
O. N. Tchaikovskaya, N. G. Bryantseva, J. L. G. Carrasco, V. S. Krayukhina, M. D. Murcia Almagro, and M. Gómez Gómez, Russ. Phys. J. 59, 552 (2016). https://doi.org/10.1007/s11182-016-0805-9
J. Wang and Sh. Wang, Chem. Eng. J. 334, 1502 (2018).
S. Waclawek, H. V. Lutze, K. Grübel, et al., Chem. Eng. J. 330, 44 (2017).
M. Khandarkhaeva, A. Batoeva, D. Aseev, et al., Ecotoxicol. Environ. Safety 137 (3), 35 (2017).
D. G. Aseev, M. R. Sizykh, and A. A. Batoeva, Russ. J. Phys. Chem. A 91, 2327 (2017).
A. Tsitonaki, B. Petry, M. Crimi, et al., Crit. Rev. Environ. Sci. Technol. 40, 55 (2010).
M. Khandarkhaeva, D. Aseev, M. R. Sizykh, and A. A. Batoeva, Russ. J. Phys. Chem. A 90, 2177 (2016).
H. Herrmann, Phys. Chem. Chem. Phys. 9, 3935 (2007).
J. Criquet, N. Karpel, and V. Leitner, Chemosphere 77, 194 (2009).
Y.-J. Shih, W. N. Putra, Y.-H. Huang, and J.-Ch. Tsai, Chemosphere 89, 1262 (2012).
G. P. Anipsitakis and D. D. Dionysiou, Appl. Catal., B 54, 155 (2004).
E. A. Sosnin, T. Oppenländer, and V. F. Tarasenko, J. Photochem. Photobiol., C 7, 145 (2006).
S. Canonica, L. Meunier, and U. Gunten, Water Res. 42, 121 (2008).
I. Epold, M. Trapido, and N. Dulova, Chem. Eng. J. 279, 452 (2015).
Y. Ji, C. Ferronato, A. Salvador, et al., Sci. Total Environ. 472, 800 (2014).
C. S. Liu, K. Shih, C. X. Sun, and F. Wang, Sci. Total Environ. 416, 507 (2012).
H. Li, J. Guo, L. Yang, and Y. Lan, Sep. Purif. Technol. 132, 168 (2014).
H. Y. Liang, Y.-G. Zhang, S.-B. Huang, and I. Hussain, Chem. Eng. J. 218, 384 (2013).
H. Kusic, I. Peternel, S. Ukic, et al., Chem. Eng. J. 172, 109 (2011).
E. Brillas and C. A. Martinez, Appl. Catal., B 166–167, 603 (2015).
F. C. Moreira, S. Garcia-Segura, V. J. P. Vilar, et al., Appl. Catal., B 142–143, 877 (2013).
M. M. Ahmed and S. Chiron, Water Res. 48, 229 (2014).
Funding
This work was performed as part of State Task no. 0339-2016-0005 for the Baikal Institute of Nature Management, Siberian Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by P. Vlasov
Rights and permissions
About this article
Cite this article
Sizykh, M.R., Batoeva, A.A. Oxidative Degradation of Azo Dyes in Combined Fenton-like Oxidative Systems. Russ. J. Phys. Chem. 93, 2349–2355 (2019). https://doi.org/10.1134/S003602441912029X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602441912029X