Abstract
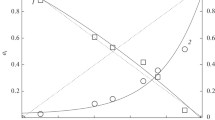
The results of studying the evaporation and thermodynamic properties of the TiO2–Al2O3 system in the temperature range 2250–2710 K by high-temperature mass spectrometry are presented. The composition of the vapor is identified, the partial pressures of the TiO2, TiO, and Al vapor species over the studied samples in the indicated temperature range are determined. The determined activities of the components and the excess Gibbs energy in the melt of the TiO2–Al2O3 system at 2345 K point to insignificant deviations from ideality.





Similar content being viewed by others
REFERENCES
Zhao, Y., Xu, H., Zhang, X., Zhu, G., Yan, D., and Yu, A., J. Eur. Ceram. Soc., 2015, vol. 35, no. 13, p. 3761. https://doi.org/10.1016/j.jeurceramsoc.2015.05.017
Tian, P., Peng, Z., Du, X., Zheng, W., and Yuan, J., Glass Phys. Chem., 2019, vol. 45, no. 3, p. 208. https://doi.org/10.1134/S1087659619030143
Sychev, M.M., Shilova, O.A., Matveichikova, P.V., Khamova, T.V., D’yachenko, S.V., Zhernovoi, A.I., and Kopitsa, G.P., Glass Phys. Chem., 2019, vol. 45, no. 6, p. 513. https://doi.org/10.1134/S1087659619060233
Shao, H., Liang, K., Zhou, F., Wang, G., and Hu, A., Mater. Res. Bull., 2005, vol. 40, no. 3, p. 499. https://doi.org/10.1016/j.materresbull.2004.11.005
Zdaniewski, W., J. Mater. Sci., 1973, vol. 8, no. 2, p. 192. https://doi.org/10.1007/BF00550667
Wange, P., Höche, T., Rüssel, C., and Schnapp, J.D., J. Non-Cryst. Solids, 2002, vol. 298, nos. 2–3, p. 137. https://doi.org/10.1016/S0022-3093(02)00950-X
Hunger, A., Carl, G., Gebhardt, A., and Rüssel, C., J. Non-Cryst. Solids, 2008, vol. 354, nos. 52–54, p. 5402. https://doi.org/10.1016/j.jnoncrysol.2008.09.001
Stolyarova, V.L. and Semenov, G.A., Mass Spectrometric Study of the Vaporization of Oxide Systems, Chichester: John Wiley, 1994.
Ilatovskaia, M., Savinykh, G., and Fabrichnaya, O., J. Phase Equilibria Diffus., 2017, vol. 38, no. 3, p. 175. https://doi.org/10.1007/s11669-016-0509-4
Kim, I.J., J. Ceram. Process. Res., 2010, vol. 11, no. 4, p. 411.
Kaufman, L., Physica. B+C, 1988, vol. 150, nos. 1–2, p. 99. https://doi.org/10.1016/0378-4363(88)90111-8
Kazenas, E.K. and Tsvetkov, Yu.V., Isparenie oksidov (Evaporation of Oxides), Moscow: Nauka,1997.
Kazenas, E.K. and Tsvetkov, Yu.V., Termodinamika ispareniya oksidov (Thermodynamics of Evaporation of Oxides), Moscow: Izd. LKI, 2008.
Bondar’, V.V., Lopatin, S.I., and Stolyarova, V.L., Inorg. Mater., 2005, vol. 41, no. 4, p. 362. https://doi.org/10.1007/s10789-005-0138-5
Gilles, P.W., Carlson, K.D., Franzen, H.F., and Wahlbeck, P.G., J. Chem. Phys., 1967, vol. 46, no. 7, p. 2461. https://doi.org/10.1063/1.1841070
Gilles, P.W., Franzen, H.F., Duane Stone, G., and Wahlbeck, P.G., J. Chem. Phys., 1968, vol. 48, no. 5, p. 1938. https://doi.org/10.1063/1.1668994
Gilles, P.W., Hampson, P.J., and Wahlbeck, P.G., J. Chem. Phys., 1969, vol. 50, no. 2, p. 989. https://doi.org/10.1063/1.1671100
Hampson, P.J. and Gilles, P.W., J. Chem. Phys., 1971, vol. 55, no. 8, p. 3708. https://doi.org/10.1063/1.1676654
Semenov, G.A., Lopatin, S.I., and Kuligina, L.A., Vestn. SPbGU, Ser. 4 (Physics, Chemistry), 1994, vol. 1, no. 4, p. 46.
Finger, L.W. and Hazen, R.M., J. Appl. Phys., 1978, vol. 49, no. 12, p. 5823. https://doi.org/10.1063/1.324598
Meagher, E.P. and Lager, G.A., Can. Mineral., 1979, vol. 17, no. 1, p. 77.
Morosin, B. and Lynch, R.W., Acta Crystallogr. B, 1972, vol. 28, no. 4, p. 1040. https://doi.org/10.1107/S0567740872003681
Lias, S.G., Bartmess, J.E., Liebman, J.F., Holmes, J.L., Levin, R.D., and Mallard, W.G., J. Phys. Chem. Ref. Data, 1988, vol. 17, no. Suppl. 1, p. 861.
Paule, R.C. and Mandel, J., Pure Appl. Chem., 1972, vol. 31, no. 3, p. 371. https://doi.org/10.1351/pac197231030371
Mann, J.B., J. Chem. Phys., 1967, vol. 46, no. 5, p. 1646. https://doi.org/10.1063/1.1840917
Drowart, J., Chatillon, C., Hastie, J., and Bonnell, D., Pure Appl. Chem., 2005, vol. 77, no. 4, p. 683. https://doi.org/10.1351/pac200577040683
Sidorov, L.N. and Shol’ts, V.B., Int. J. Mass Spectrom. Ion. Phys., 1972, vol. 8, no. 5, p. 437. https://doi.org/10.1016/0020-7381(72)80014-7
Redlich, O. and Kister, A.T., Ind. Eng. Chem., 1948, vol. 40, no. 2, p. 345. https://doi.org/10.1021/ie50458a036
Termicheskie konstanty veshchestv (Thermal Constants of Substances), Glushko, V.P., Ed., Moscow: Nauka, 1978–1982.
Barin, I., Thermochemical Data of Pure Substances, Weinheim: VCH Verlagsgesellschaft mbH, 1995.
Babushkin, V.I., Matveev, G.M., and Mchedlov-Petrosyan, O.P., Termodinamika silikatov (Thermodynamics of Silicates), Moscow: Stroiizdat, 1986.
ACKNOWLEDGMENTS
The authors are grateful to the Cryogenic Department of the Research Park of St. Petersburg State University for providing liquid nitrogen.
Funding
This work was financially supported by the Russian Foundation for Basic Research and the Science Committee of the Ministry of Education, Science, Culture and Sports of the Republic of Armenia (project no. 20-53-05013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
V.L. Stolyarova and S.I. Lopatin are members of the editorial board of the Journal of General Chemistry. No conflict of interest was declared by the other authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 10, pp. 1558–1567 https://doi.org/10.31857/S0044460X21100115.
To the 90th Anniversary of A.V. Suvorov
Rights and permissions
About this article
Cite this article
Stolyarova, V.L., Vorozhtcov, V.A., Shemchuk, D.V. et al. High Temperature Mass Spectrometric Study of the TiO2–Al2O3 System. Russ J Gen Chem 91, 1999–2007 (2021). https://doi.org/10.1134/S107036322110011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322110011X