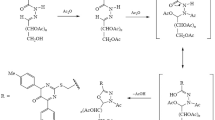
Abstract
New sugar hydrazones and their derived oxadiazolyl acyclic nucleoside analogs in addition to the derived thioglycosides incorporating pyridine, furan or thiophene have been synthesized via a multi-component reaction (MCR). The acetylated derivatives of hydrazones and the derived deprotected thioglycosides of the prepared acetylated analogs have been also synthesized. Anticancer activity of the products has been tested against adenocarcinomic human alveolar basal epithelial (A549), human prostatic adenocarcinoma (PC3) and human colorectal carcinoma (HCT116) cell lines in addition to their effect on human normal retinal pigmented epithelial cell line (RPE1) using the MTT assay. Three products: acid hydrazide, sugar hydrazine and glycoside are characterized by high activity against three cancer cell lines.



Similar content being viewed by others
REFERENCES
Li, T., Zhang, J., Pan, J., Wu, Z., Hu, D., and Song, B., Eur. J. Med. Chem., 2017, vol. 125, p. 657. https://doi.org/10.1016/j.ejmech.2016.09.069
El-Sayed, W., Khalaf, H., Mohamed, S., Hussien, H., Kutkat, O., and Amr, A., Russ. J. Gen. Chem., 2017, vol. 87, p. 2444. https://doi.org/10.1134/S1070363217100279
Nassar, I.F., El-Sayed, W.A., Ragab, T.I., Shalaby, A.S.G., and Mehany, A., Mini Rev. Med. Chem., 2019, vol. 19, p. 395. https://doi.org/10.2174/1389557518666180820125210
Stegmeier, F., Warmuth, M., Sellers, W., and Dorsch, M., Clin. Pharmacol. Ther., 2010, vol. 87, p. 543. https://doi.org/10.1038/clpt.2009.297
Fadda, A., Abdel-Rahman, A.-H., El-Sayed, W., Zidan, T., and Badria, F., Chem. Heterocycl. Compd., 2011, vol. 47, p. 856. https://doi.org/10.1007/s10593-011-0847-4
El-Essawy, F., El-Sayed, W., El-Etrawy, A.S., and El-Bayaa, M.,Chem. Heterocycl. Compd., 2013, vol. 48, p. 1853. https://doi.org/10.1007/s10593-013-1219-z
El-Sayed, W.A., Metwally, M.A., Nada, D.S., Mohamed, A.A., and Abdel-Rahman, A.A.H., J. Heterocycl. Chem., 2013, vol. 50, p. 194. https://doi.org/10.1002/jhet.901
Dewick, P.M., Medicinal Natural Products: A Biosynthetic Approach, New York: John Wiley and Sons, 2002. https://doi.org/10.1002/0470846275
Mabkhot, Y.N., Alatibi, F., El-Sayed, N.N.E., Al-Showiman, S., Kheder, N.A., Wadood, A., Rauf, A., Bawazeer, S., and Hadda, T.B., Molecules, 2016, vol. 21, p. 222. https://doi.org/10.3390/molecules21020222
Mbaveng, A.T., Sandjo, L.P., Tankeo, S.B., Ndifor, A.R., Pantaleon, A., Nagdjui, B.T., and Kuete, V., Springer Plus, 2015, vol. 4, p. 823. https://doi.org/10.1186/s40064-015-1645-8
Ajdačić, V., Senerovic, L., Vranić, M., Pekmezovic, M., Arsic-Arsnijevic, V., Veselinovic, A., Veselinovic, J., Šolaja, B.A., Nikodinovic-Runic, J., and Opsenica, I.M., Bioorg. Med. Chem., 2016, vol. 24, p. 1277. https://doi.org/10.1016/j.bmc.2016.01.058
Leitans, J., Sprudza, A., Tanc, M., Vozny, I., Zalubovskis, R., Tars, K., and Supuran, C.T., Bioorg. Med. Chem., 2013, vol. 21, p. 5130. https://doi.org/10.1016/j.bmc.2013.06.041
da Franca Rodrigues, K.A., de Sousa Dias, C.N., do Nascimento Neris, P.L., da Câmara Rocha, J., Scotti, M.T., Scotti, L., Mascarenhas, S.R., Veras, R.C., de Medeiros, I.A., and Keesen, T.D.S.L., Eur. J. Med. Chem., 2015, vol. 106, p. 1. https://doi.org/10.1016/j.ejmech.2015.10.011
Basiony, E.A., Hassan, A.A., Al-Amshany, Z.M., Abd-Rabou, A.A., Abdel-Rahman, A.A.-H., Hassan, N.A., and El-Sayed, W.A., Molecules, 2020, vol. 25, p. 399. https://doi.org/10.3390/molecules25020399
Romagnoli, R., Baraldi, P.G., Kimatrai Salvador, M., Preti, D., Aghazadeh Tabrizi, M., Bassetto, M., Brancale, A., Hamel, E., Castagliuolo, I., and Bortolozzi, R., J. Med. Chem., 2013, vol. 56, p. 2606. https://doi.org/10.1021/jm400043d
Kao, C.-L. and Chern, J.-W., Tetrahedron Lett., 2001, vol. 42, p. 1111. https://doi.org/10.1016/S0040-4039(00)02163-8
El-Sayed, W.A., Ramiz, M.M., and Adel, A.-H., Monatsh. Chem., 2008, vol. 139, p. 1499. https://doi.org/10.1007/s00706-008-0941-1
El-Sayed, W.A., Abbas, H.A.S., Mohamed, A.M., and Abdel-Rahman, A.A.H., J. Heterocycl. Chem., 2011, vol. 48, p. 1006. https://doi.org/10.1002/jhet.679
Coşkun, D., Tekin, S., Sandal, S., and Coşkun, M.F., J. Chem., 2016, vol. 2016. https://doi.org/10.1155/2016/7678486
El-Sayed, W.A. and Adel, A.-H., Z. Naturforsch. B, 2010, vol. 65, p. 57. https://doi.org/10.1515/znb-2010-0110
Ali, O.M., El-Sayed, W.A., Eid, S.A., Abdelwahed, N., and Abdel-Rahman, A., Acta Pol. Pharm., 2012, vol. 69, p. 657. PMID: 22876608
Kassem, A.F., Nassar, I.F., Abdel-Aal, M.T., Awad, H.M., and El-Sayed, W.A., Chem. Pharm. Bull., 2019, vol. 67, p. 888. https://doi.org/10.1248/cpb.c19-00280
El Ashry, E.S. and El Kilany, Y., Adv. Heterocycl. Chem., 1998, 69, p. 129. https://doi.org/10.1016/S0065-2725(08)60082-3
Rahman, A.A., Nassar, I.F., Shaban, A.K., El-Kady, D.S., Awad, H.M., and El Sayed, W.A., Mini Rev. Med. Chem., 2019, vol. 19, p. 1093. https://doi.org/10.2174/1389557519666190312165717
Alminderej, F.M., Elganzory, H.H., El-Bayaa, M.N., Awad, H.M., and El-Sayed, W.A., Molecules, 2019, vol. 24, p. 3738. https://doi.org/10.3390/molecules24203738
Kassem, A.F., Abbas, E.M., El-Kady, D.S., Awad, H.M., and El-Sayed, W.A., Mini Rev. Med. Chem., 2019, vol. 19, p. 933. https://doi.org/10.2174/1389557519666181231121217
El-Sayed, W.A., Osman, D.A., Khalaf, H.S., Abbas, H.-A.S., and Ali, M.M., Acta Pol. Pharm., 2017, vol. 74, p. 1739.
Attia, A. and Michael, M., Chemischer Informationsdienst, 1983, vol. 14. https://doi.org/10.1002/chin.198336230
Attia, A. and Kamel, M., J. Cheminf., 1988, vol. 19. https://doi.org/10.1002/chin.198806187
Mosmann, T., J. Immunol. Methods, 1983, vol. 65, p. 55. https://doi.org/10.1016/0022-1759(83)90303-4
El-Sayed, W.A., Mohamed, A.M., Khalaf, H.S., ElKady, D.S., and Al-Manawaty, M., J. Appl. Pharm., 2017, vol. 7, p. 001. https://doi.org/10.7324/JAPS.2017.70901
Vogel, A.I., Tatchell, A.R., Furnis, B.S., Hannaford, A.J., and Smith, P.W.G., Vogel’s Textbook of Practical Organic Chemistry, New York: Wiley, 1989, p. 647.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Khalaf, H.S., Tolan, H.E.M., El-Bayaa, M.N. et al. Synthesis and Anticancer Activity of New Pyridine-Thiophene and Pyridine-Furan Hybrid Compounds, Their Sugar Hydrazone, and Glycosyl Derivatives. Russ J Gen Chem 90, 1706–1715 (2020). https://doi.org/10.1134/S1070363220090182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220090182