Abstract
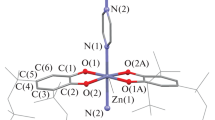
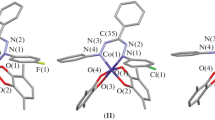
Biradical mononuclear SQ2\({\text{ZnL}}_{2}^{4}\) and SQ2ZnL5 complexes, binuclear SQ2Zn(L3)ZnSQ2 complex, and coordination polymers (SQ2ZnL1(2))n are synthesized by the reactions of bis(3,6-di-tert-butyl-o-benzosemiquinonato)zinc (SQ2Zn) with the bidentate N-donor ligands (L1–5) (pyrazine (L1), 4,4'-dipyridyl (L2), phenazine (L3), 4-cyanopyridine (L4), and pyrimidine (L5)). The structures of the synthesized compounds are determined by X-ray diffraction analysis (СIF files CCDC nos. 1846558–1846563). The binuclear SQ2Zn(L3)ZnSQ2 and mononuclear SQ2ZnL5 complexes contain pentacoordinated metal atoms, whereas zinc in the compounds based on the L1,2,4 ligands exists in the octahedral environment with the trans-arranged o-semiquinonate ligands. The coordination polymers (SQ2ZnL1(2))n are capable of depolymerizing on dissolution in organic solvents. According to the data of measuring the temperature dependence of the magnetic susceptibility, the antiferromagnetic exchange between unpaired electrons of the organic radical anions is observed in crystals of the synthesized compounds.





Similar content being viewed by others
REFERENCES
Ovcharenko, V.I. and Sagdeev, R.Z., Russ. Chem. Rev., 1999, vol. 68, p. 345.
Koivisto, B.D. and Hicks, R.G., Coord. Chem. Rev., 2005, vol. 249, p. 2612.
Ratera, I. and Veciana, J., Chem. Soc. Rev., 2012, vol. 41, p. 303.
D’Alessandro, D.M., Chem. Commun., 2016, vol. 52, p. 8957.
Jung, O.-S. and Pierpont, C.G., J. Am. Chem. Soc., 1994, vol. 116, p. 2229.
Attia, A.S. and Pierpont, C.G., Inorg. Chem., 1997, vol. 36, p. 6184.
Abakumov, G.A., Lobanov, A.V., Cherkasov, V.K., et al., Dokl. Akad. Nauk SSSR, 1985, vol. 285, p. 906.
Chen, X.-Y., Wei, R.-J., Zheng, L.-S., and Tao, J., Inorg. Chem., 2014, vol. 53, p. 13212.
Chen, L., Wei, R., Tao, J., et al., Sci. China Chem., 2012, vol. 55, p. 1037.
Imaz, I., Maspoch, D., Rodriguez-Blanco, C., et al., Angew. Chem., Int. Ed., 2008, vol. 47, p. 1857.
Drath, O., Gable, R.W., Poneti, G., et al., Cryst. Growth Des., 2017, vol. 17, p. 3156.
Drath, O., Gable, R.W., Moubaraki, B., et al., Inorg. Chem., 2016, vol. 55, p. 4141.
Cheng, W.-Q., Li, G.-L., Zhang, R., et al., J. Mol. Struct., 2015, vol. 1087, p. 68.
Goswami, S., Panja, A., Butcher, R.J., et al., Inorg. Chim. Acta, 2011, vol. 370, p. 311.
Shaikh, N., Goswami, S., Panja, A., et al., Inorg. Chem., 2005, vol. 44, p. 9714.
Glavinovic, M., Qi, F., Katsenis, A.D., et al., Chem. Sci., 2016, vol. 7, p. 707.
Gordon, A. and Ford, R., The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References, New York: Wiley, 1972.
Piskunov, A.V., Maleeva, A.V., Abakumov, G.A., et al., Russ. J. Coord. Chem., 2011, vol. 37, p. 243. https://doi.org/10.1134/S1070328411030092
Sheldrick, G.M., SHELXTL. Version 6.12. Structure Determination Software Suite, Madison: Bruker AXS, 2000.
Sheldrick, G.M., SADABS. Version 2.01. Bruker/Siemens Area Detector Absorption Correction Program, Madison: Bruker AXS, 1998.
Agilent Technologies. CrysAlis Pro, Yarnton: Agilent Technologies Ltd., 2011.
Piskunov, A.V, Lado, A.V., Abakumov, G.A. et al., Russ. Chem. Bull., 2007, vol. 56, p. 97. https://doi.org/10.1007/s11172-007-0016-1
Piskunov, A.V., Maleeva, A.V., Bogomyakov, A.S., and Fukin, G.K., Russ. Chem. Bull., 2017, vol. 66, p. 1618. https://doi.org/10.1007/s11172-017-1933-2
Ilyakina, E.V., Poddel’sky, A.I., Piskunov, A.V., et al., New J. Chem., 2012, vol. 36, p. 1944.
Piskunov, A.V., Maleeva, A.V., Bogomyakov, A.S., et al., Polyhedron, 2015, vol. 102, p. 715.
Piskunov, A.V., Maleeva, A.V., Fukin, G.K., et al., Inorg. Chim. Acta, 2017, vol. 455, p. 213.
Schmidt, R.D., Shultz, D.A., Martin, J.D., and Boyle, P.D., J. Am. Chem. Soc., 2010, vol. 132, p. 6261.
Jana, N.Ch., Brandao, P., and Panja, P., J. Inorg. Biochem., 2016, vol. 159, p. 96.
Poddels’ky, A.I., Cherkasov, V.K., and Abakumov, G.A., Coord. Chem. Rev., 2009, vol. 253, p. 291.
Brown, S.N., Inorg. Chem., 2012, vol. 51, p. 1251.
Myers, T.W., Holmes, A.L., and Berben, L.A., Inorg. Chem., 2012, vol. 51, p. 8997.
Cates, C.D., Myers, T.W., and Berben, L.A., Inorg. Chem., 2012, vol. 51, p. 11891.
Piskunov, A.V., Mescheryakova, I.N., Bogomyakov, A.S., et al., Inorg. Chem. Commun., 2009, vol. 12, p. 1067.
Piskunov, A.V., Meshcheryakova, I.N., Ershova, I.V., et al., RSC Adv., 2014, vol. 4, p. 42494.
Piskunov, A.V., Ershova, I.V., Bogomyakov, A.S., et al., Inorg. Chem., 2015, vol. 54, p. 6090.
Piskunov, A.V., Ershova, I.V., Bogomyakov, A.S., et al., Inorg. Chem. Commun., 2016, vol. 66, p. 94.
Carrington, A. and McLachlan, A., Introduction to Magnetic Resonance with Application to Chemistry and Chemical Physics, New Yoork: Harper & Row, 1967.
ACKNOWLEDGMENTS
The physicochemical studies of the compounds were carried out using the equipment of the Analytical Center of the Razuvaev Institute of Organometallic Chemistry (Russian Academy of Sciences).
Funding
This work was supported by the Russian Science Foundation, project no. 14-13-01296.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Piskunov, A.V., Maleeva, A.V., Bogomyakov, A.S. et al. Bis-o-Semiquinonate Zinc Complexes with the Bidentate N-Donor Ligands: Synthesis and Magnetic Properties. Russ J Coord Chem 45, 309–319 (2019). https://doi.org/10.1134/S1070328419050026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419050026