Abstract
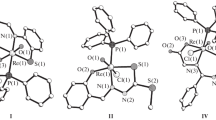
The structural features of eleven mononuclear octahedral d2-Re(V) monooxo complexes with tridentate chelating (O,S,O and S,O,S) ligands, \(\left[ {{\text{ReO}}\left( {\text{L}_{\text{tri}}^{m}} \right)\left( {\text{L}_{\text{bi}}^{n}} \right)} \right],\)\(\left[ {{\text{ReO}}\left( {\text{L}_{\text{tri}}^{m}} \right){\text{C}}{{{\text{l}}}_{{\text{2}}}}} \right]\), are discussed. The \({\text{Re-O}}{{\left( {\text{L}_{\text{tri}}^{m}} \right)}_{{trans}}}\) bond lengths (except for two cases) were shown to be similar to (or somewhat shorter than), the Re–O(L)cis or Re–O(ST) bond lengths, which is indicative of the presence of pseudo-dioxo ReO2 groups with increased bond orders for both trans-arranged Re–O bonds. In the structures of two compounds, the \({\text{Re-O}}{{\left( {\text{L}_{\text{tri}}^{m}} \right)}_{{trans}}}\) bonds are, on average, 0.093 Å longer than the Re–O(ST) bonds, which is consistent with the structural requirements of the trans-effect of a multiply bonded oxo ligand.


Similar content being viewed by others
REFERENCES
Porai-Koshits, M.A. and Gilinskaya, E.A., Kristallokhimiya (Crystallochemistry), Moscow: VINITI, 1966, p. 126.
Porai-Koshits, M.A. and Atovmyan, L.O., Koord. Khim., 1975, vol. 1, p. 1271.
Griffith, F. and Wicing, C., J. Chem. Soc. A, 1968, p. 379.
Porai-Koshits, M.A., Izv. Yugoslav. Kristallogr. Tsentra, 1974, vol. 9, p. 19.
Porai-Koshits, M.A. and Atovmyan L.O., Kristallokhimiya koordinatsionnykh soedinenii molibdena (Crystal Chemistry of Coordination Compounds of Molybdenum), Moscow: Nauka, 1974.
Shustorovich, E.M., Porai-Koshits, M.A., and Buslaev, Yu.A., Coord. Chem. Rev., 1975, vol. 17, p. 1.
Porai-Koshits, M.A. and Sergienko, V.S., Usp. Khim., 1990, vol. 59, p. 86.
Allen, F.H., Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, p. 380.
Sergienko, V.S., Russ. J. Inorg. Chem., 2018, vol. 63, no. 14, p. 1757. doi 10.1134/S0036023618140048
Sergienko, V.S., Russ. J. Inorg. Chem., 2016, vol. 61, p. 1408. doi 10.1134/S0036023616110188
Sergienko, V.S. and Churakov, A.V., Crystallogr. Rep., 2014, vol. 59, no. 3, p. 300. doi 101134/S106377451140301711
Sergienko, V.S. and Churakov, A.V., Crystallogr. Rep., 2013, vol. 58 no. 1, p. 5. https://doi.org/10.1134/S1063774513010112
Femia, F.J., Babich, J.W., and Zubieta, J., Inorg. Chim. Acta, 2000, vols. 300−302, p. 462.
Babich, J.W., Graham, W., Femia, F.J., et al., Inorg. Chim. Acta, 2001, vol. 323, p. 23.
Al-Jeboori, M.J., Dilworth, J.R., and Hiller, W., Inorg. Chim. Acta, 1999, vol. 285, p. 76.
Pietzsch, H.-J., Reisgys, M., Spis, H., et al., Chem. Ber., 1997, vol. 130, p. 357.
Pietzsch, H.T., Polyhedron, 1995, vol. 14, p. 1849.
Reo, T.N., Adhikesovaly, D., Camerman, A., and Fitzkerg, A.R., J. Chem. Soc., 1990, vol. 112, p. 5798.
Powell, J.L., Inorg. Chim. Acta, 1995, vol. 229, p. 241.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Sergienko, V.S., Churakov, A.V. The Structure of Monomeric Octahedral d2-Rhenium(V) Monooxo Complexes \(\left[ {{\mathbf{ReO}}\left( {{\mathbf{L}}_{{{\mathbf{tri}}}}^{m}} \right)\left( {{\mathbf{L}}_{{{\mathbf{bi}}}}^{n}} \right)} \right],\)\(\left[ {{\mathbf{ReO}}\left( {{\mathbf{L}}_{{{\mathbf{tri}}}}^{m}} \right){\mathbf{C}}{{{\mathbf{l}}}_{{\mathbf{2}}}}} \right]\) with Oxygen Atoms of Tridentate Chelating (O,S,O and S,O,S) Ligands. Russ J Coord Chem 45, 326–332 (2019). https://doi.org/10.1134/S1070328419030072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419030072