Abstract
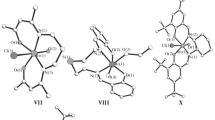
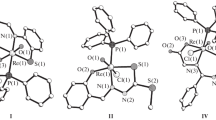
The structural features of six mononuclear octahedral monooxo d2-Re(V) complexes [ReO(Lmono)\(\left( {{\text{L}}_{{{\text{tetra}}}}^{n}} \right)\)] with tetradentate chelate (O, Х3; Х = O, N, P) \(\left( {{\text{L}}_{{{\text{tetra}}}}^{n}} \right)\) and monodentate (Lmono) ligands are discussed. The O\(\left( {{\text{L}}_{{{\text{tetra}}}}^{n}} \right)\) atoms are always located in the trans positions to the multiply bound O(oxo) ligands.


Similar content being viewed by others
REFERENCES
M. A. Porai-Koshits and E. A. Gilinskaya, Itogi Nauki Tekh.: Kristallokhimiya, 126 (1966).
M. A. Porai-Koshits and L. O. Atovmyan, Koord. Khim. 1, 1271 (1975).
F. Griffith and C. Wicing, J. Chem. Soc. A, No. 3, 379 (1968).
M. A. Porai-Koshits, Izv. Yugosl. Kristallogr. Tsentra 9, 19 (1974).
M. A. Porai-Koshits and L. O. Atovmyan, The Crystallochemistry of Molybdenum Coordination Compounds (Nauka, Moscow, 1974) [in Russian].
E. M. Shustorovich, M. A. Porai-Koshits, and Yu. A. Buslaev, Coord. Chem. Rev. 17, 1 (1975).
M. A. Porai-Koshits and V. S. Sergienko, Usp. Khim. 59 (1), 86 (1990).
F. H. Allen, Acta Crystallogr. B 58, 380 (2002).
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 61, 1708 (2016).
V. S. Sergienko, Russ. J. Inorg. Chem. 62, 751 (2017).https://doi.org/10.1134/S0036023617060195
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 62, 1327 (2017). https://doi.org/10.1134/S0036023617100151
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 63, 631 (2018). https://doi.org/10.1134/S0036023618050121
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 63, 753 (2018). https://doi.org/10.1134/S0036023618060219
V. S. Sergienko, Russ. J. Inorg. Chem. 63, 1757 (2018).
V. S. Sergienko and A. V. Churakov, Russ. J. Coord. Chem. 45, 332 (2019). https://doi.org/10.1134/S1070328419030072
V. S. Sergienko and A. V. Churakov, Russ. J. Coord. Chem. 45, 439 (2019).
V. S. Sergienko and A. V. Churakov, Russ. J. Coord. Chem. (in press).
V. S. Sergienko and A. V. Churakov, Crystallography Repts 59, 300 (2014).
V. S. Sergienko and A. V. Churakov, Crystallography Repts 58, 5 (2013). https://doi.org/10.1134/S106377451301010112
J. M. Botha, K. Umakoshi, Y. Sasaki, and G. J. Lamprecht, Inorg. Chem. 37, 1609 (1998).
T. I. A. Gerber, P. Mayer, and Z. R. Tshentu, J. Coord. Chem. 60, 237 (2007).
T. I. A. Gerber, P. Mayer, and Z. R. Tshentu, J. Coord. Chem. 56, 1357 (2003).
T. I. A. Gerber, P. Mayer, and Z. R. Tshentu, J. Coord. Chem. 58, 947 (2005).
M. Allali, E. Benoist, M. Gressier, et al., Dalton Trans., No. 9, 3178 (2004).
A. Barandov and U. Abram, Inorg. Chem. 48, 8072 (2009).
C. Redshaw, X. Liu, S. Zhan, et al., Eur. J. Inorg. Chem, No. 10, 2698 (2008).
ACKNOWLEDGMENTS
The author is grateful to A.V. Churakov for his help in searching for data from Cambridge Structural Database.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Sergienko, V.S. Structural Features of Monomeric Octahedral Monooxo d2-Rhenium(V) Complexes [ReO(Lmono)(\(L_{{{\text{tetra}}}}^{n}\))] with Oxygen Atoms of Tridentate Chelate Ligands OХ3, Х = O, N, or P (\(L_{{{\text{tetra}}}}^{n}\)). Russ. J. Inorg. Chem. 64, 1127–1131 (2019). https://doi.org/10.1134/S0036023619090183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619090183