Abstract
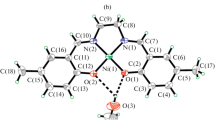
Two mononuclear nickel(II) complexes, [Ni(L1)2] (1) and [Ni(L2)2] (2), where L1 and L2 are the anions of 4-methylbenzoic acid (1-pyridin-2-ylmethylidene)hydrazide (HL1) and benzoic acid (1-pyridine-2-ylethylidene)hydrazide (HL2), respectively, were prepared and characterized by physico-chemical methods and single crystal X-ray diffraction. The tridentate Schiff base ligands coordinate to the Ni atoms through the pyridine nitrogen, imino nitrogen and enolate oxygen atoms. The Ni atom in each complex is six coordinated by two Schiff base ligands, to form octahedral coordination. To investigate the influence of the position of methyl group on the antibacterial activities, the complexes have been studied on the bacteria Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas fluorescens.





Similar content being viewed by others
REFERENCES
Y. Chen, P. Li, S. J. Su, M. Chen, J. He, L. W. Liu, M. He, H. Wang, and W. Xue. RSC Adv., 2019, 9, 23045. https://doi.org/10.1039/c9ra05139b
R. Fekri, M. Salehi, A. Asadi, and M. Kubicki. Inorg. Chim. Acta, 2019, 484, 245. https://doi.org/10.1016/j.ica.2018.09.022
D. A. Sabbah, F. Al-Tarawneh, W. H. Talib, K. Sweidan, S. K. Bardaweel, E. Al-Shalabi, H. A. Zhong, G. Abu Sheikha, R. Abu Khalaf, and M. S. Mubarak. Med. Chem., 2018, 14, 695. https://doi.org/10.2174/1573406414666180412160142
H. B. Zhou, C. Chen, Y. S. Liu, and X. P. Shen. Inorg. Chim. Acta, 2015, 437, 188. https://doi.org/10.1016/j.ica.2015.08.020
M. Yadav, V. Mereacre, S. Lebedkin, M. M. Kappes, A. K. Powell, and P. W. Roesky. Inorg. Chem., 2015, 54, 773. https://doi.org/10.1021/ic5014957
M. Alexandru, M. Cazacu, A. Arvinte, S. Shova, C. Turta, B. C. Simionescu, A. Dobrov, E. C. B. A. Alegria, L. M. D. R. S. Martins, A. J. L. Pombeiro, and V. B. Arion. Eur. J. Med. Chem., 2014, 1, 120. https://doi.org/10.1002/ejic.201300969
K. Jana, S. Das, H. Puschmann, S. C. Debnath, A. Shukla, A. K. Mahanta, M. Hossain, T. Maity, and B. C. Samanta. Inorg. Chim. Acta, 2019, 487, 128. https://doi.org/10.1016/j.ica.2018.12.007
F. Forouzandeh, H. Keypour, M. H. Zebarjadian, M. Mahmoudabadi, L. Hosseinzadeh, R. Karamian, M. A. Khoei, and R. W. Gable. Polyhedron, 2019, 160, 238. https://doi.org/10.1016/j.poly.2018.12.052
M. H. Esfahani, H. Iranmanesh, J. E. Beves, M. Kaur, J. P. Jasinski, and M. Behzad. J. Coord. Chem., 2019, 72, 2326. https://doi.org/10.1080/00958972.2019.1643846
M. Zhang, D.-M. Xian, H.-H. Li, J.-C. Zhang, and Z.-L. You. Aust. J. Chem., 2012, 65, 343. https://doi.org/10.1071/CH11424
P. G. Avaji, C. H. V. Kumar, S. A. Patil, K. N. Shivananda, and C. Nagaraju. Eur. J. Med. Chem., 2009, 44, 3552. https://doi.org/10.1016/j.ejmech.2009.03.032
M. J. Hearn, M. H. Cynamon, M. F. Chen, R. Coppins, J. Davis, H. J. O. Kang, A. Noble, B. Tu-Sekine, M. S. Terrot, D. Trombino, M. Thai, E. R. Webster, and R. Wilson. Eur. J. Med. Chem., 2009, 44, 4169. https://doi.org/10.1016/j.ejmech.2009.05.009
A. Datta, N.-T. Chuang, and J.-H. Huang. J. Chem. Crystallogr., 2011, 41, 1780. https://doi.org/10.1007/s10870-011-0173-9
A. Datta, N.-T. Chuang, M.-H. Sie, J.-H. Huang, and H. M. Lee. Acta Crystallogr., Sect. E, 2010, 66, m359. https://doi.org/10.1107/S1600536810007336
Bruker. SMART and SAINT. Madison, WI: 2002.
G. M. Sheldrick. SADABS. Program for Empirical Absorption Correction of Area Detector. Germany: University of Göttingen, 1996.
G. M. Sheldrick. SHELXTL V5.1 Software Reference Manual. Madison, WI: Bruker AXS, 1997.
H.-H. Li, Z.-L. You, C.-L. Zhang, M. Yang, L.-N. Gao, and L. Wang. Inorg. Chem. Commun., 2013, 29, 118. https://doi.org/10.1016/j.inoche.2012.12.023.
Z.-L. You, M. Zhang, and D.-M. Xian. Dalton Trans., 2012, 41, 2515. https://doi.org/10.1039/c1dt11566a
Y. Luo, J. Wang, X. Ding, R. Ni, M. Li, T. Yang, J. Wang, C. Jing, and Z. You. Inorg. Chim. Acta, 2021, 516, 120146. https://doi.org/10.1016/j.ica.2020.120146
X.-M. Hu, L.-W. Xue, G.-Q. Zhao, and Y.-J. Han. Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2012, 42, 557. https://doi.org/10.1080/15533174.2011.613884
L.-W. Xue, X. Wang, and G.-Q. Zhao. Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2012, 42, 1334. https://doi.org/10.1080/15533174.2012.680139
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 6, pp. 823-825.https://doi.org/10.26902/JSC_id94089
Rights and permissions
About this article
Cite this article
Sang, YL., Lin, XS., Zou, LF. et al. SYNTHESES, CRYSTAL STRUCTURES AND ANTIBACTERIAL ACTIVITIES OF MONONUCLEAR NICKEL(II) COMPLEXES WITH SIMILAR SCHIFF BASES. J Struct Chem 63, 956–963 (2022). https://doi.org/10.1134/S0022476622060130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622060130