Abstract
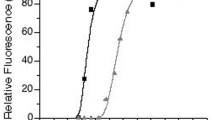
Among the wide variety of currently known fluorescent probes, photoconvertible fluorescent proteins (PCFPs), which are capable to irreversibly change their maximum of fluorescence emission under specific light irradiation, are of special interest. In this work, we have studied the basic physicochemical properties of PCFP isolated from Montastraea cavernosa (mcavGR). It has been shown that mcavGR demonstrates both high brightness and photoconversion contrast, which is comparable with the best homologous PCFPs, such as Kaede, dendFP, Dendra2, EosFP, etc., and at the same time has significant photostability.




Similar content being viewed by others
REFERENCES
Chudakov, D.M., Matz, M.V., Lukyanov, S., and Lukyanov, K.A., Physiol. Rev. Am. Physiol. Soc., 2010, vol. 90, pp. 1103–1163. https://doi.org/10.1152/physrev.00038.2009
Martynov, V.I., Pakhomov, A.A., Popova, N.V., Deyev, I.E., and Petrenko, A.G., Acta Naturae, 2016, vol. 8, pp. 33–46.
Tomosugi, W., Matsuda, T., Tani, T., Nemoto, T., Kotera, I., Saito, K., Horikawa, K., and Nagai, T., Nat. Methods, 2009, vol. 6, pp. 351–353. https://doi.org/10.1038/nmeth.1317
Shinoda, H., Ma, Y., Nakashima, R., Sakurai, K., Matsuda, T., and Nagai, T., Cell Chem. Biol., 2018, pp. 330e7–338e7. https://doi.org/10.1016/j.chembiol.2017.12.005
Pakhomov, A.A. and Martynov, V.I., Biochem. Biophys. Res. Commun., 2011, vol. 407, pp. 230–235. https://doi.org/10.1016/j.bbrc.2011.03.004
Pakhomov, A.A., Frolova, A.Y., Tabakmakher, V.M., Chugunov, A.O., Efremov, R.G., and Martynov, V.I., J. Photochem. Photobiol. B, 2020, vol. 206, p. 111 853. https://doi.org/10.1016/j.jphotobiol.2020.111853
Fabritius, A., Ng, D., Kist, A.M., Erdogan, M., Portugues, R., and Griesbeck, O., Cell Chem. Biol., 2018, vol. 25, pp. 1554e8–1561e8. https://doi.org/10.1016/j.chembiol.2018.08.008
Kostyuk, A.I., Demidovich, A.D., Kotova, D.A., Belousov, V.V., and Bilan, D.S., Int. J. Mol. Sci., 2019, vol. 20, p. 4200. https://doi.org/10.3390/ijms20174200
Martynov, V.I., Pakhomov, A.A., Deyev, I.E., and Petrenko, A.G., Biochim. Biophys. Acta—Gen. Subj., 2018, vol. 1862, pp. 2924–2939.
Bukhari, H. and Muller, T., Trends Cell Biol., 2019, vol. 29, pp. 912–928. https://doi.org/10.1016/j.tcb.2019.08.004
Mozhaev, A.A., Serova, O.V., Orsa, A.N., Boyko, A.A., Goryashchenko, A.S., Deyev, I.E., and Petrenko, A.G., Russ. J. Bioorg. Chem., 2019, vol. 45, pp. 179–182. https://doi.org/10.1134/S1068162019020080
Zlobovskaya, O.A., Shirmanova, M.V., Kovaleva, T.F., Sarkisyan, K.S., Zagaynova, E.V., and Lukyanov, K.A., Russ. J. Bioorg. Chem., 2018, vol. 44, pp. 645–652. https://doi.org/10.1134/S1068162018060109
Adam, V., Histochem. Cell Biol., 2014, vol. 142, pp. 19–41. https://doi.org/10.1007/s00418-014-1190-5
Nemet, I., Ropelewski, P., and Imanishi, Y., Photochem. Photobiol. Sci., 2015, vol. 14, pp. 1787–1806. https://doi.org/10.1039/C5PP00174A
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H., and Miyawaki, A., Proc. Natl. Acad. Sci. U. S. A., 2002, vol. 99, pp. 12 651–12 656. https://doi.org/10.1073/pnas.202320599
Fron, E., Sliwa, M., Adam, V., Michiels, J., Rocha, S., Dedecker, P., Hofkens, J., and Mizuno, H., Photochem. Photobiol. Sci., 2014, vol. 13, pp. 867–874. https://doi.org/10.1039/c3pp50335f
Mizuno, H., Mal, T.K., Tong, K.I., Ando, R., Furuta, T., Ikura, M., and Miyawaki, A., Mol. Cell, 2003, vol. 12, pp. 1051–1058. https://doi.org/10.1016/S1097-2765(03)00393-9
Li, X., Chung, L.W., Mizuno, H., Miyawaki, A., and Morokuma, K., J. Phys. Chem. B, vol. 114, pp. 16 666–16 675. https://doi.org/10.1021/jp1101779
Chudakov, D.M., Lukyanov, S., and Lukyanov, K.A., Nat. Protoc., 2007, vol. 2, pp. 2024–2032. https://doi.org/10.1038/nprot.2007.291
Baker, S.M., Buckheit, R.W., and Falk, M.M., BMC Cell Biol., 2010, vol. 11, p. 15. https://doi.org/10.1186/1471-2121-11-15
Hoi, H., Shaner, N.C., Davidson, M.W., Cairo, C.W., Wang, J., and Campbell, R.E., J. Mol. Biol., 2010, vol. 401, pp. 776–791. https://doi.org/10.1016/j.jmb.2010.06.056
Moeyaert, B., Holt, G., Madangopal, R., Perez-Alvarez, A., Fearey, B.C., Trojanowski, N.F., Ledderose, J., Zolnik, T.A., Das, A., Patel, D., Brown, T.A., Sachdev, R.N.S., Eickholt, B.J., Larkum, M.E., Turrigiano, G.G., Dana, H., Gee, C.E., Oertner, T.G., Hope, B.T., and Schreiter, E.R., Nat. Commun., 2018, vol. 9, p. 4440. https://doi.org/10.1038/s41467-018-06935-2
Osugi, T., Sasakura, Y., and Satake, H., Sci. Rep., vol. 10, p. 1892. https://doi.org/10.1038/s41598-020-58884-w
Betzig, E., Patterson, G.H., Sougrat, R., Lindwasser, O.W., Olenych, S., Bonifacino, J.S., Davidson, M.W., Lippincott-Schwartz, J., and Hess, H.F., Science, 2006, vol. 313, pp. 1642–1645. https://doi.org/10.1126/science.1127344
McKinney, S.A., Murphy, C.S., Hazelwood, K.L., Davidson, M.W., and Looger, L.L., Nat. Methods, 2009, vol. 6, pp. 131–133. https://doi.org/10.1038/nmeth.1296
Nienhaus, K. and Ulrich Nienhaus, G., Chem. Soc. Rev., 2014, vol. 43, pp. 1088–1106. https://doi.org/10.1039/C3CS60171D
Robert, A., Hookway, C., and Gelfand, V.I., BioEssays, 2016, vol. 38, pp. 232–243. https://doi.org/10.1002/bies.201500142
Habuchi, S., Tsutsui, H., Kochaniak, A.B., Miyawaki, A., and van Oijen, A.M., PLoS One, 2008, vol. 3, pp. 1–9. https://doi.org/10.1371/journal.pone.0003944
Adam, V., Moeyaert, B., David, C.C., Mizuno, H., Lelimousin, M., Dedecker, P., Ando, R., Miyawaki, A., Michiels, J., Engelborghs, Y., and Hofkens, J., Chem. Biol., 2011, vol. 18, pp. 1241–1251.
McEvoy, A.L., Hoi, H., Bates, M., Platonova, E., Cranfill, P.J., Baird, M.A., Davidson, M.W., Ewers, H., Liphardt, J., and Campbell, R.E., PLoS One, 2012, vol. 7, e51 314. https://doi.org/10.1371/journal.pone.0051314
Labas, Y.A., Gurskaya, N.G., Yanushevich, Y.G., Fradkov, A.F., Lukyanov, K.A., Lukyanov, S.A., and Matz, M.V., Proc. Natl. Acad. Sci. U. S. A., 2002, vol. 99, pp. 4256–4261. https://doi.org/10.1073/pnas.062552299
Wiedenmann, J., Ivanchenko, S., Oswald, F., Schmitt, F., Rocker, C., Salih, A., Spindler, K.-D., and Nienhaus, G.U., Proc. Natl. Acad. Sci. U. S. A., 2004, vol. 101, pp. 15 905–15 910. https://doi.org/10.1073/pnas.0403668101
Pakhomov, A.A., Martynova, N.Yu., Gurskaya, N.G., Balashova, T.A., and Martynov, V.I., Biochemistry (Moscow), 2004, vol. 69, pp. 901–908. https://doi.org/10.1023/B:BIRY.0000040223.09641.29
Ando, R., Mizuno, H., and Miyawaki, A., Science, 2004, vol. 306, pp. 1370–1373. https://doi.org/10.1126/science.1102506
Karasawa, S., Araki, T., Yamamoto-Hino, M., and Miyawaki, A., J. Biol. Chem., 2003, vol. 278, pp. 34 167–34 171. https://doi.org/10.1074/jbc.M304063200
Tsutsui, H., Karasawa, S., Shimizu, H., Nukina, N., and Miyawaki, A., EMBO Rep., 2005, vol. 6, pp. 233–238. https://doi.org/10.1038/sj.embor.7400361
Ai, H.W., Biochem. J., 2006, vol. 400, pp. 531–540.
Kelmanson, I.V. and Matz, M.V., Mol. Biol. Evol., 2003, vol. 20, pp. 1125–1133. https://doi.org/10.1093/molbev/msg130
Field, S.F., Bulina, M.Y., Kelmanson, I.V., Bielawski, J.P., and Matz, M.V., J. Mol. Evol., 2006, vol. 62, pp. 332–339. https://doi.org/10.1007/s00239-005-0129-9
Kim, H., Grunkemeyer, T.J., Modi, C., Chen, L., Fromme, R., Matz, M.V., and Wachter, R.M., Biochemistry, 2013, vol. 52, pp. 8048–8059. https://doi.org/10.1021/bi401000e
Kim, H., Zou, T., Modi, C., Dorner, K., Grunkemeyer, T.J., Chen, L., Fromme, R., Matz, M.V., Ozkan, S.B., and Wachter, R.M., Structure, 2015, vol. 23, pp. 34–43. https://doi.org/10.1016/j.str.2014.11.011
Mazel, C.H., Lesser, M.P., Gorbunov, M.Y., Barry, T.M., Farrell, J.H., Wyman, K.D., and Falkowski, P.G., Limnol. Oceanogr., 2003, vol. 48, pp. 402–411. https://doi.org/10.4319/lo.2003.48.1_part_2.0402
Lambert, T.J., Nat. Methods, 2019, vol. 16, pp. 277–278. https://doi.org/10.1038/s41592-019-0352-8
Pakhomov, A.A., Chertkova, R.V., and Martynov, V.I., Russ. J. Bioorg. Chem., 2015, vol. 41, pp. 602–606. https://doi.org/10.1134/S1068162015060114
Pletneva, N.V., Pletnev, S., Pakhomov, A.A., Chertkova, R.V., Martynov, V.I., Muslinkina, L., Dauter, Z., and Pletnev, V.Z., Acta Crystallogr. Sect. D Struct. Biol., 2016, vol. 72, pp. 922–932. https://doi.org/10.1107/S205979831601038X
Tsutsui, H., Shimizu, H., Mizuno, H., Nukina, N., Furuta, T., and Miyawaki, A., Chem. Biol., 2009, vol. 16, pp. 1140–1147. https://doi.org/10.1016/j.chembiol.2009.10.010
Paez-Segala, M.G., Sun, M.G., Shtengel, G., Viswanathan, S., Baird, M.A., Macklin, J.J., Patel, R., Allen, J.R., Howe, E.S., Piszczek, G., Hess, H.F., Davidson, M.W., Wang, Y., and Looger, L.L., Nat. Methods, 2015, vol. 12, pp. 215–218. https://doi.org/10.1038/nmeth.3225
Pakhomov, A.A., Martynov, V.I., Orsa, A.N., Bondarenko, A.A., Chertkova, R.V., Lukyanov, K.A., Petrenko, A.G., and Deyev, I.E., Biochem. Biophys. Res. Commun., 2017, vol. 493, pp. 1518–1521. https://doi.org/10.1016/j.bbrc.2017.09.170
Katoh, K. and Toh, H., Bioinformatics, 2007, vol. 23, pp. 372–374. https://doi.org/10.1093/bioinformatics/btl592
Gouy, M., Guindon, S., and Gascuel, O., Mol. Biol. Evol., 2010, vol. 27, pp. 221–224. https://doi.org/10.1093/molbev/msp259
Guindon, S., Dufayard, J.-F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O., Syst. Biol., 2010, vol. 59, pp. 307–321. https://doi.org/10.1093/sysbio/syq010
Orm, M., Cubitt, A.B., Kallio, K., Gross, L.A., Tsien, R.Y., and Remington, S.J., Science, 1996, vol. 273, pp. 1392–1395. https://doi.org/10.1126/science.273.5280.139
Baird, G.S., Zacharias, D.A., and Tsien, R.Y., Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, pp. 11 984–11 989. https://doi.org/10.1073/pnas.97.22.11984
Ward, W.W., Green Fluorescent Protein: Properties, Applications, and Protocols, 2nd ed., Chalfie, M. and Kain, S.R., Eds., Wiley, 2005, pp. 39–65. https://doi.org/10.1002/0471739499.ch3
Sniegowski, J.A., Phail, M.E., and Wachter, R.M., Biochem. Biophys. Res. Commun., 2005, vol. 332, pp. 657–663. https://doi.org/10.1016/j.bbrc.2005.04.166
Hoops, S., Sahle, S., Gauges, R., Lee, C., Pahle, J., Simus, N., Singhal, M., Xu, L., Mendes, P., and Kummer, U., Bioinformatics, 2006, vol. 22, pp. 3067–3074. https://doi.org/10.1093/bioinformatics/btl485
ACKNOWLEDGMENTS
The authors thank K.A. Lukyanov from the Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences for providing plasmids that carried the gene of the mcavGR fluorescent protein.
Funding
The work was supported by grants nos. 18-04-00745 and 18-29-09166 from the Russian Foundation for Basic Research and, partially, grant no. 19-73-20194 from Russian Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies with the use of humans as objects of research.
Conflict of Interests
The authors state that there is no conflict of interest.
Additional information
Translated by A. Levina
Abbreviations: FP, fluorescent protein; PCFP, Photoconvertible Fluorescent Protein; dendFP, PCFP from Dendronephthya sp.; Dendra2, monomeric mutant variant of dendFP; Kaede, PCFP from Trachyphyllia geoffroyi; mcavGR, mcavRFP, PCFP from Montastraea cavernosa.
Corresponding author: phone: +7 (495) 336-51-11; fax: +7 (495) 336-61-66.
Rights and permissions
About this article
Cite this article
Frolova, A.Y., Pakhomov, A.A. & Martynov, V.I. Physicochemical Properties of Photoconvertible Fluorescent Protein from Montastraea cavernosa . Russ J Bioorg Chem 47, 244–251 (2021). https://doi.org/10.1134/S1068162021010052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021010052