Abstract
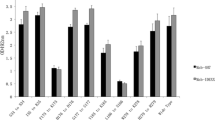
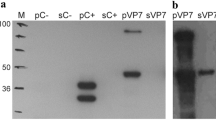
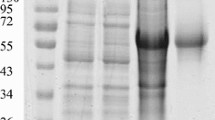
Bluetongue, or catarrhal fever of sheep, is a viral vector-borne infection of ruminants, which is one of the economically significant arbovirus infections of animals. It is transmitted by blood-sucking insects of the Culicoides genus. Viral protein VP7 is a group-specific core protein of the virus, conservative for all known serotypes, and therefore represents the most suitable target for creation of diagnostic tests. To develop an immunochemical method for detecting viral infection, a panel of high-affinity monoclonal antibodies to VP7 has been obtained. The N-terminal fragment of VP7 expressed in E. coli and inactivated viral particles were used as immunogens. The resulting monoclonal antibodies are useful for detecting the virus in infected cells. As a species-specific antigen, a recombinant TrxA‑VP7_a protein has been created that contains spatial epitopes similar to those of the native viral antigen. With its help, promising antibodies were selected for diagnostics of the disease by competitive enzyme-linked immunosorbent assay, which allows the detection of specific antibodies to bluetongue virus in the sera of infected animals. Using competitive solid-phase ELISA, it has been shown that antibodies that interact most effectively with the TrxA-VP7_a protein (Bt14, Bt15, Bt18, Bt26, Bt33, Bt34, and Bt35) recognize close or overlapping VP7 epitopes. The interaction with the antigen of almost all the antibodies obtained was inhibited by reference specific sera against the bluetongue virus of 24 serotypes. The monoclonal antibody Bt14 showed maximum ability to block the interaction of VP7 with specific sera against 24 bluetongue virus serotypes. Thus, the resulting panel of monoclonal antibodies to VP7 of the bluetongue virus can be used to detect viral infection, as well as a component of kits for serological diagnosis of the disease.


Similar content being viewed by others
REFERENCES
Mertens, P.P., Diprose, J., Maan, S., Singh, K.P., Attoui, H., and Samuel, A.R., Vet. Ital., 2004, vol. 40, pp. 426–437.
Makarov, V.V., Shoopala, D., Dzhupina, S.I., and Sukharev, O.I., Vet. Segodnya, 2013, vol. 1, pp. 8–10.
Mellor, P.S., Baylis, M., Peter, P.C., and Mertens, P.P., London: Academic, 2009.
Ratinier, M., Caporale, M., Golder, M., Franzoni, G., Allan, K., Nunes, S.F., Armezzani, A., Bayoumy, A., Rixon, F., Shaw, A., and Palmarini, M., PLoS Pathog., 2011, vol. 7, p. 1 002 477.
Mertens, P.P., Brown, F., and Sangar, D.V., Virology, 1984, vol. 135, pp. 207–217.
Van Dijk, A.A. and Huismans, H., J. Gen. Virol., 1988, vol. 69, part 3, pp. 573–581.
Savini, G., Goffredo, M., Monaco, F., Di Gennaro, A., de Santis, P., Meiswinkel, R., and Caporale, V., Vet. Ital., 2004, vol. 40, pp. 286–291.
Meiswinkel, R., Baldet, T., De Deken, R., Takken, W., Delecolle, J.C., and Mellor, P.S., Prev. Vet. Med., 2008, vol. 87, pp. 55–63.
Zakharov, V.M., Veterinariya, 2009, vol. 5, pp. 3–5.
Makarov, V.V., Sukharev, O.I., and Vasilevich, F.I., Veterinariya, 2014, no. 6, pp. 18–23.
Hewat, E.A., Booth, T.F., Wade, R.H., and Roy, P., J. Struct. Biol., 1992, vol. 108, pp. 35–48.
Afshar, A., Comp. Immunol. Microbiol. Infect. Dis., 1994, vol. 17, nos. 3–4, pp. 221–242.
Koumbati, M., Mangana, O., Nomikou, K., Mellor, P.S., and Papadopoulos, O., Vet. Microbiol., 1999, vol. 64, pp. 277–285.
Mars, M.H., van Maanen, C., Vellema, P., Kramps, J.A., and van Rijn, P.A., Vet. Microbiol., 2010, vol. 146, pp. 209–214.
Wu, X., Liu, Q., He, J., Zang, M., Wang, H., Li, Y., and Tang, L., Monoclon. Antib. Immunodiagn. Immunother., 2015, vol. 34, pp. 116–121.
Wilson, W.C., Ma, H.C., Venter, E.H., van Djik, A.A., and Seal, B.S., Virus Res., 2000, vol. 67, pp. 141–151.
Wang, L.F., Hyatt, A.D., Whiteley, P.L., Andrew, M., Li, J.K., and Eaton, B.T., Arch. Virol., 1996, vol. 141, pp. 111–123.
Verwoerd, D.W., Els, H.J., De Villiers, E.M., and Huismans, H., J. Virol., 1972, vol. 10, pp. 783–794.
Kohler, G. and Milstein, C., Nature, 1975, vol. 256, pp. 495–497.
La Vallie, E.R., Di Blasio, E.A., Kovacic, S., Grant, K.L., Schendel, P.F., and McCoy, J.M., Biotechnology (New York), 1993, vol. 2, pp. 187–193.
Stevens, R.C., Structure, 2000, vol. 8, pp. 177–185.
Balysheva, V.I. and Novikova, M.B., A method of obtaining a universal inactivated bluetongue virus antigen for serodiagnosis, RF Patent no. 2 352 357.
Baumgarten, H. and Franze, R., in Monoclonal Antibodies, Peters, J.H. and Baumgarten, H., Eds., Springer-Verlag, 1992, pp. 223–233, 264–271.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, pp. 248–254.
Hay, F.C. and Westwood, O.M.R., Practical Immunology, 4th ed., London: Wolverhampton, Blackwell Science, 2002.
Beatty, J.D., Beatty, B.G., and Vlahos, W.G., J. Immunol. Methods, 1987, vol. 100, pp. 173–179.
Wilson, M.B. and Nakane, P.K., in Immunofluorescence and Related Staining Techniques, Knapp, W., Wick, G., and Holubar, K., Eds., Amsterdam: Elsevier–Academic, 1978, pp. 215–224.
Karatovskaya, A., Rudenko, N., Tsfasman, I., Guseva, K., Laman, A., Boziev, K., Brovko, F., and Vasilyeva, N., Proc. Biochem. Soc., 2016, vol. 51, pp. 1521–1526.
Funding
This work was carried out in the framework of a state contract no. 0101-2019-0038 “New Biomaterials and Bionanotechnologies for Diagnostics and Therapy” and a state contract no. 0615-2019-0003 under the auspices of the Basic Research Program of State Academies of Sciences 159.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
In carrying out this work, all ethical standards were observed.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Statement of the Welfare of Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
This article does not contain any studies involving human participants performed by any of the authors.
Additional information
Translated by A. Ostyak
Abbreviations: mAb, monoclonal antibodies; mAb-HRP, mAb conjugated to horseradish peroxidase; iIHC-ELISA, indirect Immunohistochemical enzyme-linked immunosorbent assay; spELISA, solid phase enzyme-linked immunosorbent assay; TCID, tissue culture infectious dose; SK, saiga kidney cell culture; BTV, bluetongue virus; CV-1, normal African Green Monkey kidney fibroblast cell lineage; FCS, fetal calf serum; N, interaction of mAb-HRP with immobilized TrxA-VP7_a in the absence of competing antibodies; PBS, phosphate buffered saline; PBST, PBS containing 0.1% Tween 20; S, interaction of mAb-HRP with immobilized TrxA-VP7_a in the presence of competing antibodies; Vero, normal African Green Monkey kidney fibroblast cell lineage.
Corresponding author: phone: +7 (926) 592-11-89; e-mail: nrudkova@mail.ru.
Rights and permissions
About this article
Cite this article
Rudenko, N.V., Karatovskaya, A.P., Shepelyakovskaya, A.O. et al. Monoclonal Antibodies to the Recombinant Protein VP7 of Bluetongue Virus. Russ J Bioorg Chem 45, 505–513 (2019). https://doi.org/10.1134/S1068162019060347
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162019060347