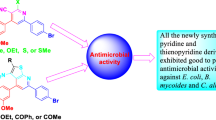
Abstract
A series of new N-benzoyl-N'-triazine thiourea derivatives have been synthesized via the reaction of 4-amino-6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one with benzoyl chloride derivatives and ammonium thiocyanate in acetone under reflux conditions. 4-Amino-6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one was prepared from the reaction of two equivalents of hydrazine hydrate with carbon disulfide and sodium pyruvate. The chemical structure of thioureas was confirmed using FT-IR, 1H NMR, 13C NMR, and high-resolution mass spectrometry, and elemental analysis. The synthesized thioureas were assayed for their antibacterial activity against both gram-positive (Micrococcusluteus and Bacilluscereus) and gram-negative (Pseudomonasaeruginosa and Escherichiacoli) bacteria using the agar well diffusion method.


Similar content being viewed by others
REFERENCES
Binzet, G., Arslan, H., Florke, U., Kulcu, N., and Duran, N., J. Coord. Chem., 2006, vol. 59, pp. 1395−1406.
Ugur, D., Arslan, H., and Kulcu, N., Russ. J. Coord. Chem., 2006, vol. 32, pp. 669−675.
Emen, M.F., Arslan, H., Kulcu, N., Florke, U., and Duran, N., Pol. J. Chem., 2005, vol. 79, pp. 1615−1626.
Arslan, H., Florke, U., Kulcu, N., and Emen, M.F., J. Coord. Chem., 2006, vol. 59, pp. 223−228.
Yang, W., Hu, Y., Yang, Y.S., Zhang, F., Zhang, Y.B., Wang, X.L., Tang, J.F., Zhong, W.Q., and Zhu, H.L., Bioorg. Med. Chem., 2013, vol. 21, pp. 1050−1063.
Shantharam, C.S., Suyoga, V.D.M., Suhas, R., Sridhara, M.B., and Channe, G.D., Eur. J. Med. Chem., 2013, vol. 60, pp. 325−332.
Celen, A.O., Kaymakcioglu, B., Gumru, S., Toklu, H.Z., and Aricioglu, F., Marmara Pharm. J., 2011, vol. 15, pp. 43−47.
Huang, X.C., Wang, M., Pan, Y.M., Yao, G.Y., Wang, H.S., Tian, X.Y., Qin, J.K., and Zhang, Y., Eur. J. Med. Chem., 2013, vol. 69, pp. 508−520.
Lambert, W.T., Goldsmith M.E., and Sparks, T.C., Pest. Manag. Sci., 2017, vol. 73, pp. 743−751.
Eweis, M., Elkholy, S.S., and Elsabee, M.Z., Int. J. Biol. Macromol., 2006, vol. 38, pp. 1−8.
Tuncel, S.T., Gunal, S.E., Ekizoglu, M., Kelekci, N.G., Erdem, S.S., Bulak, E., Frey, W., and Dogan, I., J. Mol. Str., 2019, vol. 1179, pp. 40−56.
Kechea, A.P., Hatnapurea, G.D., Talec, R.H., Rodgec, A.H., and Kamble, V.M., Bioorg. Med. Chem. Lett., 2012, vol. 22, pp. 6611−6615.
Khatri, N., Lather, V., and Madan, A.K., Chemom. Intell. Lab. Syst., 2015, vol. 140, pp. 13−21.
Perveen, S., Fatima, N., Aitmaud Khan, M., Dar, A., Khan, K.M., Afza, N., and Voelter, W., Med. Chem. Res., 2012, vol. 21, pp. 2709−2715.
Beasley, S.C., Cooper, N., Gowers, L., Gregory, J.P., Haughan, A.A.F., Hellewell, P.G., Macar, D., Miotla, J., Montana, J.G., Morgan, T., Taylor, R., Runcie, K.A., and Tuladhar, B., Bioorg. Med. Chem. Lett., 1998, vol. 8, pp. 2629−2634.
Weide, T., Saldanha, S.A., Minond, D., Spicer, T.P., Fotsing, J.R., Spaargaren, M., Frere, J.M., Bebrone, C., Sharpless, K.B., Hodder, P.S., and Fokin, V.V., Acs Med. Chem. Lett., 2010, vol. 1, pp. 150−154.
Pingaew, R., Prachayasittikul, V., Anuwongcharoen, N., Prachayasittikul, S., Ruchirawat, S., and Prachayasittikul, V., Bioorg. Chem., 2018, vol. 79, pp. 171−178.
Zaib, S., Saeed, A., Stolte, K., Florke, U., Shahid, M., and Iqbal, J., Eur. J. Med. Chem., 2014, vol. 78, pp. 140−150.
Saeed, A., Zaib, S., Pervez, A., Mumtaz, A., Shahid, M., and Iqbal, J., Med. Chem. Res., 2013, vol. 22, pp. 3653−3662.
Tuomilehto, J., Lindstrom, J., Eriksson, J.G., Valle, T.T., Hamalainen, H., Ilanne-Parikka, P., Keinanen-Kiukaanniemi, S., Laakso, M., Louheranta, A., Rastas, M., and Salminen, V., N. Engl. J. Med., 2001, vol. 344, pp. 1343−1350.
Hu, F.B., Manson, J.E., Stampfer, M.J., Colditz, G., Liu, S., Solomon, C.G., and Willett, W.C.N., Engl. J. Med., 2001, vol. 345, pp. 790−797.
Samir, Y.A., Sh. El-Sharief Marwa, A.M., Wahid, M.B., Issa, M.I.F., and El-G. Eman, W., Eur. J. Med. Chem., 2013, vol. 64, pp. 111−120.
Faidallah, H.M., Al-Saadi, M.S., Rostom, S.A.F., and Fahmy, H.T.Y., Med. Chem. Res., 2007, vol. 16, pp. 300−318.
Rao, X.P., Wu, Y., Song, Z.Q., Shang, S.B., and Wang, Z.D., Med. Chem. Res., 2011, vol. 20, pp. 333−338.
Jin, L., Qu, H.E., Huang, X.C., Pan, Y.M., Liang, D., Chen, Z.F., Wang, H.S., and Zhang, Y., Int. J. Mol. Sci., 2015, vol. 16, pp. 14571−14593.
Zhang, C., Wang, X., Liu, H.C., Zhang, M.M., Geng, M.Y., Sun, L.P., Shen, A.J., and Zhang, A., Eur. J. Med. Chem., 2017, vol. 125, pp. 315−326.
Wei, Q., Ning, J.Y., Dai, X., Gao, Y.D., Su, L., Zhao, B.X., and Miao, J.Y., Eur. J. Med. Chem., 2018, vol. 145, pp. 551−558.
Tabatabaee, M., Ghassemzadeh, M., Zarabi, B., and Neumuller, B., Z. Naturforsch., 2006, vol. 61b, pp. 1421−1425.
Ghanim, A.M., Knight, D.W., Osman, N.A., Abdel-Fattah, H.A., and Kadry, A.M., Tetrahedron Lett., 2016, vol. 57, pp. 2215−2218.
Kaushik, D., Ahmad Khan, S., and Chawla, G., Eur. J. Med. Chem., 2010, vol. 45, pp. 3960−3969.
Mamolo, M.G., Falagiani, V., Zampieri, D., Vio, L., and Banfi, E., II Farmaco, 2000, vol. 55, pp. 590−595.
Sweeney, M., Coyle, R., Kavanagh, P., Berezin, A.A., Re, D.L., Zissimou, G.A., Koutentis, P.A., Carty, M.P., and Aldabbagh, F., Bioorg. Med. Chem., 2016, vol. 24, pp. 3565−3570.
Dolzhenko, A.V., Tan, B.J., Chiu, G.N.C., Chui, W.K., and Dolzhenko, A.V., J. Fluorine Chem., 2015, vol. 175, pp. 68−72.
Pourshamsian, K. and Ojani, S., Planta. Med., 2016, vol. 82, PC62.
Pourshamsian, K., Chem. Solid Mater., 2015, vol. 2, pp. 1−9.
Khakiani, A., Pourshamsian, K., and Veisi, H., Appl. Organomet. Chem., 2015, vol. 29, pp. 259-265.
Pourshamsian, K., Int. J. Bio-Inorg. Hybr. Nanomater., 2015, vol. 4, pp. 225−231.
Pourshamsian, K., Int. J. Nano. Dimens., 2015, vol. 6, pp. 99−104.
Adhami, F., Nabilzadeh, N., Emmerling, F., Ghiasi, M., and Heravi, M.M., J. Serb. Chem. Soc., 2012, vol. 77, pp. 1211−1222.
Mohammadi Zeydi, M., Montazeri, N., and Fouladi, M., J. Heterocycl. Chem., 2017, vol. 54, pp. 3549−3553.
Zamani, H.A., Rajabzadeh, G., Firouz, A., and Ariaii-Rad, A.A., J. Braz. Chem. Soc., 2005, vol. 16, pp. 1061−1067.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Additional information
Corresponding author: e-mail: Kshams49@gmail.com (Kh. Pourshamsian); abolfazl.shiroudi@iauet.ac.ir (A. Shiroudi).
Rights and permissions
About this article
Cite this article
Marzi, M., Pourshamsian, K., Hatamjafari, F. et al. Synthesis of New N-Benzoyl-N'-Triazine Thiourea Derivatives and Their Antibacterial Activity. Russ J Bioorg Chem 45, 391–397 (2019). https://doi.org/10.1134/S106816201905008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816201905008X