Abstract
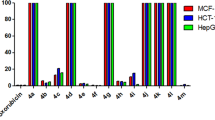
6-(Benzo[d]thiazol-2-yl)-4-oxo-(4H)-chromene-3-carbaldehyde was utilized to construct a novel series of fused chromone bearing benzothiazole moiety, namely chromeno[2,3-b]azetol, benzo[c]chromene, and chromeno[2,3-c]pyrazole derivatives, as well as, non-fused chromones, such as thiazolidinone, pyrazolone, and pyridine derivatives. The in vitro antitumor activities of the synthesized products against six cancer cell lines, including A594, HCT-116, MCF-7, HepPG2, PC3, and HFB4, were evaluated. Some compounds exhibited significant anticancer activities against both lung and colon cancer cells very close to that of a standard drug doxorubicin.

Similar content being viewed by others
REFERENCES
Gottesman, M.M., Annu. Rev. Med., 2002, vol. 53, pp. 615–627.
Nobili, S., Landini, I., Giglioni, B., and Mini, E., Curr. Drug Targets, 2006, vol. 7, no. 7, pp. 861–879.
Li, J., Wang, X.L., Fang, Y.C., and Wang, C.Y., J. Asian Nat. Prod. Res., 2010, vol.12, no. 11, pp. 992–1000.
Patil, S. A., Wang, J., Li, X.S., Chen, J., Jones, T.S., Hosni-Ahmed, A., Patil, R., Seibel, W.L., Li, W., and Miller, D.D., Bioorg. Med. Chem. Lett., 2012, vol. 22, no. 13, pp. 44–58.
Kandeel, M.M., Kamal, A.M., Abdelall, E.K.A., and Elshemy, H.A.H., Der Pharma Chemica, 2012, vol. 4, no. 4, pp. 1653–1661.
Thomas, N. and Zachariah, S.M., Asian J. Pharm. Clin. Res., 2013, vol. 6, suppl. 2, pp. 11–15.
Zonouzi, A., Mirzazadeh, R., Safavi, M., Ardestani, S.K., Emami, S., and Foroumadi, A., Iranian J. Pharm. Res. (IJPR),2013, vol. 12, no. 4, pp. 679–685.
Reddy, K.R., Rao, P.S., Dev, G.J., Poornachandra, Y., Kumar, C.G., Rao, P.S., and Narsaiah, B., Bioorg. Med. Chem. Lett., 2014, vol. 24, no. 7, pp. 1661–1663.
Rahmani-Nezhad, S., Safavi, M., Pordeli, M., Ardestani, S.K., Khosravani, L., Pourshojaei, Y., Mahdavi, M., Emami, S., Foroumadi, A., and Shafiee, A., Eur. J. Med. Chem., 2014, vol. 86, pp. 562–569.
Fouad, S.A., Int. J. Adv. Res., 2014, vol. 2, no. 12, pp. 442–453.
Banerjee, S., Wang, J., Pfeffer, S., Ma, D., Pfeffer, L.M., Patil, S.A., Li, W., and Miller, D.D., Molecules, 2015, vol. 20, pp. 17152–17165.
Puppala, M., Zhao, X., Casemore, D., Zhou, B., Aridoss, G., Narayanapillai, S., and Xing, C., Bioorg. Med. Chem., 2016, vol. 24, no. 6, pp. 1292–1297.
Fouda, A.M., Med. Chem. Res., 2016, vol. 25, pp. 1229–1238.
Salem, M.S., Marzouk, M.I., Ali, S.N., and Madkour, H.M. F., Eur. J. Chem., 2012, vol. 3, no. 2, pp. 220–227.
Yadav, P.S., Devprakash, and Senthilkumar, G. P., Int. J. Pharm. Sci. Drug Res., 2011, vol. 3, no. 1, pp. 1–7.
Abbas, E.M.H., Salem, M.S., Kassem, A.F.M., Abd El-Moez, Sh.I., and El-Kady, M., Eur. J. Chem., 2015, vol. 6, no. 2, pp. 98–106.
Kok, S.H., Gambari, R., Chui, C.H., Yuen, M.C.W., Lin, E., Wong, R.S.M., Lau, F.Y., Cheng, G.Y.M., Lam, W.S., Chan, S.H., Lam, K.H., Cheng, C.H., Lai, P.B.S., Yu, M.W.Y., Cheung, F., Tang, J.C.O.,and Chan, A.S.C., Bioorg. Med. Chem., 2008, vol. 16, no. 7, pp. 3626–3631.
Havrylyuk, D., Mosula, L., Zimenkovsky, B., Vasylenko, O., Gzella, A., and Lesyk, R., Eur. J. Med. Chem., 2010, vol. 45, no. 11, pp. 5012–5021.
Devmurari, V.P., Shivanand, P., Goyani, M.B., Nandanwar, R.R., Jivani, N.P., and Perumal, P. Int. J. Chem. Tech. Res., 2010, vol. 2, no. 1, pp. 681–689.
Furlan, A., Colombo, F., Kover, A., Issaly, N., Tintori, C., Angeli, L., Leroux, V., Letard, S., Amat, M., Asses, Y., Maigret, B., Dubreuil, P., Botta, M., Dono, R., Bosch, J., Piccolo, O., Passarella, D., and Maina, F., Eur. J. Med. Chem., 2012, vol. 47, pp. 239–254.
Shi, X., Wang, Z., Xia, Y., Ye, T., Deng, M., Xu, Y., Wei, Y., and Yu, L., Molecules, 2012, vol. 17, pp. 3933–3944.
Caputo, R., Calabrò, M.L., Micale, N., Schimmer, A.D., Ali, M., Zappalà, M., and Grasso, S., Med. Chem. Res., 2012, vol. 21, no. 9, pp. 2644–2651.
Lindgren, E.B., de Brito, M.A., Vasconcelos, T.R.A., de Moraes, M.O., Montenegro, R.C., Yoneda, J.D., and Leal, K.Z., Eur. J. Med. Chem., 2014, vol. 86, pp. 12–16.
Gurdal, E.E., Buclulgan, E., Durmaz, V., Cetin-Atalay, R., and Yarim, M., Anticancer Agents Med. Chem., 2015, vol. 15, no. 3, pp. 382–389.
El-Damasy, A.K., Lee, J.H., Seo, S.H., Cho, N.C., Pae, A.N., and Keum, G., Eur. J. Med. Chem., 2016, vol. 115, pp. 201–216.
Singh, M., Singh, S.K., Thakur, B., Ray, P., and Singh, S.K., Anticancer Agents Med. Chem., 2016, vol. 16, no. 6, pp. 722–739.
Meenakshi, S., Arusha, M., Gopeshwar, N., and Sushil, S.K., Anti-Cancer Drugs, 2016, vol. 27, no. 6, pp. 519–532.
Mohamed, K.S., Refat, H.M., and Mohamed, N.A.H., Heterocycles, 2016, vol. 92, no. 8, pp. 1415–1429.
Bozdag-Dundar, O., Evranos, B., Das-Evcimen, N., Sarhkaya, M., and Ertan, R., Eur. J. Med. Chem., 2008, vol. 43, no. 11, pp. 2412–2417.
Havrylyuk, D., Zimenkovsky, B., Vasylenko, O., Day, C.W., Smee, D.F., Grellier, P., and Lesyk, R., Eur. J. Med. Chem., 2013, vol. 66, pp. 228–237.
Kapoor, G., Pathak, D.P., Bhutani, R., and Kant, R., J. Chem. Pharm. Res., 2016, vol. 8, no. 4, pp. 151–168.
Salem, M.S., and Ali, M.A.M., Biol. Pharm. Bull., 2016, vol. 39, pp. 473–483.
El-Hashash, M.A.M., Salem, M.S., and Al-Mabrook, S.A.M., Res. Chem. Intermed., 2018, vol. 44, pp. 2545–2559.
Pal, D., Saha, S., and Singh, S., Int. J. Pharm. Pharm. Sci., 2012, vol. 4, no. 2, pp. 98–104.
Jamwal, A., Javed, A., and Bhardwaj, V., J. Pharm. Bio. Sci., 2014, vol. 3, pp. 114–123.
Ali, A.R., El-Bendary, E.R., Ghaly, M.A., and Shehata, I.A., Eur. J. Med. Chem., 2014, vol. 75, pp. 492–500.
Lacova, M., Loos, D., Furdik, M., Matulova, M., and El-Shaaer, H.M., Molecules, 1998, vol. 3, pp. 149–158.
Hu, K., Yang, Z., Pan, S., Xu, H., and Ren, J., Eur. J. Med. Chem., 2010, vol. 45, pp. 3453–3458.
Altıntop, M.D., Temel, H.E., Sever, B., Çiftçi, G.A., and Kaplancıkl, Z.A., Molecules, 2016, vol. 2, pp. 1598–1617.
Hassan, G., El-Messry, S., Al-Omary, F., and El-Sabbagh, H., Bioorg. Med. Chem. Lett., 2012, vol. 22, no. 20, pp. 6318–6323.
Amit, C., Sheelmani, S.A., Payal, C., and Dhawan, R.K. Adv. J. Pharm. Life Sci. Res., 2014, vol. 2, no. 1, pp. 1–7.
Spanò, V., Attanzio, A., Cascioferro, S., Carbone, A., Montalbano, A., Barraja, P., Tesoriere, L., Cirrincione, G., Diana, P., and Parrino, B., Mar. Drugs, 2016, vol. 14, pp. 226–244.
Das, D., Banerjee, R., and Mitra, A., J. Chem. Pharm. Res., 2014, vol. 6, no. 11, pp. 108–116.
Silva, M.S., Pinto, D.C.G.A., Cavaleiro, J.A.S., Levai, A., and Patonay, T., Arkivoc, 2004, vol. VII, pp. 106–123.
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J.T., Bokesch, H., Kenney, S., and Boyd, M.R., J. Nati. Cancer Inst., 1990, vol. 82, pp. 1107–1112.
ACKNOWLEDGMENTS
Authors would like to express their deep appreciation and indebtedness to Chemistry Department, Faculty of Science, Ain Shams University, for their support of the work.
Author information
Authors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
The article is published in the original.
Corresponding author: e-mail: marwa_k@sci.asu.edu.eg.
Rights and permissions
About this article
Cite this article
Eman A. El-Helw, Derbala, H.A., El-Shahawi, M.M. et al. Synthesis and In Vitro Antitumor Activity of Novel Chromenones Bearing Benzothiazole Moiety. Russ J Bioorg Chem 45, 42–53 (2019). https://doi.org/10.1134/S1068162019010047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162019010047