Abstract
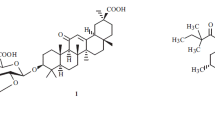
The formation of self-associates of glycyrrhetinic acid (GLA), an aglycone of glycyrrhizic acid (GA), has been studied by electrospray ionization mass spectrometry. It has been shown for the first time that, analogously to triterpene saponins having free carboxyl groups, GLA stereoisomers can form self-associates. The capacity of GLA to form self-associates has been confirmed by the mass spectrometry data. It has been found that the ionization of GLA self-associates with the formation of an anion proceeds rather weakly. The ionization of GLA in the positive ion mode goes on effectively and makes it possible to record multidimensional structures of one to eight 18α-GLA molecules and from one to nine 18β-GLA molecules. The structure of GLA associates and their stability are demonstrated most comprehensively in positive ion mass spectra. As a whole, the sets and intensity of peaks for 18α- and 18β-GLA correlate well. The results of the mass spectrometry study show the capacity of 18α- and 18β-GLA stereoisomers to form self-associates, which indicates a high potentiality of GLA in providing noncovalent interactions during the formation of supramolecular complexes. Similarly to the saponins of licorice and ivy, GLA stereoisomers may form a potential basis for the synthesis of a new generation of noncovalent molecular complexes and novel highly effective medicinal substances owing to a possible improvement in bioaccessibility and possible synergistic effects.
Similar content being viewed by others
Abbreviations
- GLA:
-
glycyrrhetinic acid
- GA:
-
glycyrrhizic acid
- EIS-MS:
-
electrospray ionization mass spectrometry
References
Tolstikov, G.A., Baltina, L.A., Shul’ts, E.E., and Pokrovskii, A.G., Bioorg. Khim., 1997, vol. 23, no. 9, pp. 691–703.
Tolstikova, T.G., Tolstikov, A.G., and Tolstikov, G.A., Vestn. Ross. Akad. Nauk, 2007, vol. 77, no. 10, pp. 867–874.
Li, H.-E., Qiu, J.-Z., Yang, Z.-Q., Dong, J., Wang, J.-F., Luo, M.-J., Pan, J., Dai, X.-H., Zhang, Y., Song, B.-L., and Deng, X.-M., Fitoterapiya, 2012, vol. 83, no. 1, pp. 241–248.
Lai, Y., Shen, L., Zhang, Z., Liu, W., Zhang, Y., Ji, H., and Tian, J., Bioorg. Med. Chem. Lett., 2010, vol. 20, no. 22, pp. 6416–6420.
Yamaguchi, H., Noshita, T., Yu, T., Kidachi, Y., Kamiie, K., Umetsu, H., and Ryoyama, K., Eur. J. Med. Chem., 2010, vol. 45, no. 7, pp. 2943–2948.
Krausse, R., Bielenberg, J., Blaschek, W., and Ullmann, U., J. Antimicrob. Chemother., 2004, vol. 54, no. 1, pp. 243–246.
Zhou, X., Zhao, L., Liu, X., Li, X., Jia, F., Zhang, Y., and Wang, Y., Phytother. Res., 2012, vol. 26, pp. 253–258.
Borisenko, S.N., Lekar’, A.V., Milov, A.A., Vetrova, E.V., and Borisenko, N.I., Khim. Rastit. Syr’ya, 2013, no. 2, pp. 85–92.
Lekar’, A.V., Vetrova, E.V., Borisenko, N.I., Yakovishin, L.A., and Grishkovets, V.I., Khim. Rastit. Syr’ya, 2011, no. 2, pp. 103–106.
Lekar’, A.V., Vetrova, E.V., Borisenko, N.I., Yakovishin, L.A., and Grishkovets, V.I., Russ. J. Bioorg. Chem., 2012, vol. 38, no. 7, pp. 749–752.
Lekar’, A.V., Yakovishin, L.A., Vetrova, E.V., Rudnev, M.I., and Borisenko, N.I., J. Anal. Chem., 2011, vol. 66, no. 13, pp. 44–48.
Borisenko, S.N., Lekar’, A.V., Vetrova, E.V., and Borisenko, N.I., Chem. Nat. Comp., 2013, vol. 49, no. 5, pp. 969–971.
Tolstikova, T.G., Sorokina, I.V., Bryzgalov, A.O., Lifshits, G.I., and Khvostov, M.V., Biomeditsina, 2006, no. 4, pp. 115–117.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.N. Borisenko, A.V. Lekar’, E.V. Vetrova, O.V. Filonova, N.I. Borisenko, 2015, published in Khimiya Rastitel’nogo Syr’ya, 2015, No. 1, pp. 89–94.
Rights and permissions
About this article
Cite this article
Borisenko, S.N., Lekar’, A.V., Vetrova, E.V. et al. A mass spectrometry study of the self-association of glycyrrhetinic acid molecules. Russ J Bioorg Chem 42, 716–720 (2016). https://doi.org/10.1134/S1068162016070037
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162016070037