Abstract
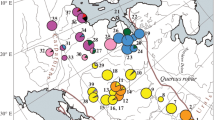
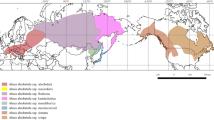
Chloroplast DNA variability was examined in 872 trees of pedunculate oak (Q. robur L.), sessile oak (Q. petraea (Matt.) Liebl.) and downy oak (Q. pubescens Willd.) on the Crimean Peninsula, in the Western Caucasus and in the Balkan region in order to study phylogeography and interaction of these species in the Black Sea region. Sequencing of five fragments with a total length of more than 10,000 base pairs revealed 12 haplotypes of chloroplast DNA. For the haplotype typing in the studied populations, chloroplast microsatellites (cpSSR), sequencing, and restriction analysis were used. Haplotypes detected belong to several divergent phylogenetic lineages. The studied species almost do not differ from each other in the composition of haplotypes and the geographical structure of variability, which demonstrates a certain level of gene flow between them in mixed populations. The haplotypes of the Balkan region are closely related to the haplotypes of previously studied populations from Eastern Europe and the western part of the Russian Plain, and are not found in the Crimea and the Caucasus. On the Crimean Peninsula, two geographical groups of populations are distinguished, which differ sharply in the composition of haplotypes. The difference between the western part of the peninsula and the eastern part is shown, which suggests a multiple origin of oak populations in the Crimea as a result of migrations from two sources, which could be facilitated by fluctuations in the Black Sea level and its desalination, which repeatedly occurred in the Pleistocene and Holocene. The predominance of two divergent haplotypes in the western part of the peninsula, similar to the haplotypes of Asia Minor, indicates the penetration of oak from this region and the presence of an isolated refugium in the mountainous forest regions of Crimea during the last glacial maximum. At the same time, haplotypes common with the Western Caucasus are spread in the east of the mountain-forest part of the Eastern Crimea. The sharp boundary between the areas of distribution of “western” and “eastern” haplotypes in the Eastern Crimea indicates a relatively recent time of the formation of a secondary contact zone between local and Caucasian oak populations as a result of postglacial colonization.



Similar content being viewed by others
Change history
01 November 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S1067413623550015
REFERENCES
Didukh, Ya.P., Rastitel’nyi pokrov gornogo Kryma (struktura, dinamika, evolyutsiya i okhrana) (Vegetation Covers of the Mountainous Crimea (Structure, Dynamics, Evolution and Protection)), Kyiv: Naukova Dumka, 1992.
Yena, A.V., Prirodnaya flora Krymskogo poluostrova (Spontaneous Flora of the Crimean Peninsula), Simferopol: N. Orianda, 2012.
Dufresnes, C., Litvinchuk, S.N., Leuenberger, J., et al., Evolutionary melting pots: A biodiversity hotspot shaped by ring diversifications around the Black Sea in the Eastern tree frog (Hyla orientalis), Mol. Ecol., 2016, vol. 25, pp. 4285–4300. https://doi.org/10.1111/mec.13706
Ekhvaia, J., Simeone, M.C., Silakadze, N., and Abdaladze, O., Morphological diversity and phylogeography of the Georgian durmast oak (Q. petraea subsp. iberica) and related Caucasian oak species in Georgia (South Caucasus), Tree Genet. Genom., 2018, vol. 14, no. 2, pp. 17. https://doi.org/10.1007/s11295-018-1232-6
Tekpinar, A.D., Aktas, C., Kansu, C., et al., Phylogeography and phylogeny of genus Quercus L. (Fagaceae) in Turkey implied by variations of trnT((UGU))-L-(UAA)-F ((GAA)) chloroplast DNA region, Tree Genet. Genom., 2021, vol. 17, no. 5, p. 40. https://doi.org/10.1007/s11295-021-01522-x
Kukushkin, O.V., Ermakov, O.A., Ivanov, A.Yu., et al., Cytochrome b mitochondrial gene analysis-based phylogeography of a sand lizard in the Crimea: Ancient refugium at the peninsula, late expansion from the North, and first evidence of Lacerta agilis tauridica and L. a. exigua (Lacertidae: Sauria) hybridization, Proceedings Zool. Inst. Ross. Akad. Nauk, 2020, vol. 324, no. 1, pp. 56–99. https://doi.org/10.31610/trudyzin/2020.324.1.56
Kukushkin, O., Ermakov, O., Gherghel, I., et al., The mitochondrial phylogeography of the Crimean endemic lizard Darevskia lindholmi (Sauria, Lacertidae): Hidden diversity in an isolated mountain system, Vertebr. Zool., 2021, vol. 71, pp. 559–576. https://doi.org/10.3897/vz.71.e62729
Petit, R.J., Csaikl, U.M., Bordacs, S., et al., Chloroplast DNA variation in European white oaks—phylogeography and patterns of diversity based on data from over 2600 populations, For. Ecol. Manage., 2002, vol. 156, nos. 1–3, pp. 5–26. https://doi.org/10.1016/S0378-1127(01)00645-4
Petit, R.J., Brewer, S., Bordacs, S., et al., Identification of refugia and postglacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence, For. Ecol. Manage., 2002, vol. 156, pp. 49–74. https://doi.org/10.1016/S0378-1127(01)00634-X
Semerikova, S.A., Isakov, I.Yu, and Semerikov, V.L., Chloroplast DNA variation and phylogeography of pedunculate oak Quercus robur L. in the eastern part of the range, Russ. J. Genet., 2021, vol. 57, no. 1, pp. 47–60. https://doi.org/10.1134/S1022795421010130
Degen, B., Yanbaev, Y., Mader, M., et al., Impact of gene flow and introgression on the range wide genetic structure of Quercus robur (L.) in Europe, Forests, 2021, vol. 12, no. 10, p. 1425. https://doi.org/10.3390/f12101425
Takhtajan, A.L., Floristicheskie oblasti Zemli (The Floristic Regions of the World), Leningrad: Nauka, 1978.
Garkusha, L.Ya., Bagrova, L.A., and Pozachenyuk, E.A., Diversity of Crimean landscapes with Mediterranean elements of flora, Uch. Zap. Tavricheskogo Nats. Univ. im. V.I. Vernadskogo, Ser. Geogr., 2012, vol. 25, no. 2, pp. 36–47.
Lesa SSSR (Forests of the USSR), Moscow: Nauka, 1966, vol. 3.
Plugatar', Yu.V., Lesa Kryma (Crimean Forests), Simferopol: Arial, 2015.
Kremer, A. and Hipp, A.L., Oaks: An evolutionary success story, New Phytol., 2020, vol. 226, no. 4, pp. 987–1011. https://doi.org/10.1111/nph.16274
Menitsky, Y.L., Oaks of Asia, Enfield, NH: Science, 2005.
Curtu, A.L., Gailing, O., and Finkeldey, R., Evidence for hybridization and introgression within a species-rich oak (Quercus spp.) community, BMC Evol. Biol., 2007, vol. 7, p. 218. https://doi.org/10.1186/1471-2148-7-218
Semerikov, L.F., Populyatsionnaya struktura drevesnykh rastenii (na primere vidov duba evropeiskoi chasti SSSR i Kavkaza) (Population Structure of Trees: The Example of Oak Species in the European Soviet Union and the Caucasus), Moscow: Nauka, 1986.
Gerasimenko, N., Environmental changes in the Crimean mountains during the Last Interglacial–Middle Pleniglacial as recorded by pollen and lithopedology, Quat. Int., 2007, vol. 164–165, pp. 207–220. https://doi.org/10.1016/j.quaint.2006.12.018
Gerasimenko, N.P., Bezusko, L.G., Avdieienko, Y.L., and Yanevich, A.A., Late Glacial and Holocene vegetational and climate changes and their impact on material cultures in the Crimean Mountains (founded on pollen data from cave deposits), Quat. Int., 2022, vol. 632, no. 20, pp. 139–153. https://doi.org/10.1016/j.quaint.2021.12.018
Cordova, C.E., Gerasimenko, N.P., Lehman, P.H., and Kliukin, A.A., Late Pleistocene and Holocene paleoenvironments of Crimea: Pollen, soils, geomorphology, and geoarchaeology, in Geology and Geoarchaeology of the Black Sea Region: Beyond the Flood Hypothesis, Buynevich, I.V., Yanko-Hombach, V., Gilbert, A.S., and Martin, R.E., Eds., Geol. Soc. Am. Special Paper, 2011, vol. 473, pp. 133–164. https://doi.org/10.1130/2011.2473(09)
Markova, A.K., Small mammals from Palaeolithic of the Crimea, Quat. Int., 2011, vol. 231, nos. 1–2, pp. 22–27. https://doi.org/10.1016/j.quaint.2010.07.016
Cameron, R.A.D., Pokryszko, B.M., and Horsak, M., Forest snail faunas from Crimea (Ukraine), an isolated and incomplete Pleistocene refugium, Biol. J. Linn. Soc., 2013, vol. 109, pp. 424–433. https://doi.org/10.1111/bij.12040
Krijgsman, W., Tesakov, A., Yanina, T., et al., Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution, Earth-Sci. Rev., 2019, vol. 188, pp. 1–40. https://doi.org/10.1016/j.earscirev.2018.10.013
Doan, K., Mackiewicz, P., Sandoval-Castellanos, E., et al., The history of Crimean red deer population and Cervus phylogeography in Eurasia, Zool. J. Linn. Soc., 2018, vol. 183, no. 2, pp. 208–225. https://doi.org/10.1093/ZOOLINNEAN/ZLX065
Mayol, M., Riba, M., González-Martínez, S.C., et al., Adapting through glacial cycles: insights from a long-lived tree (Taxus baccata), New Phytol., 2015, vol. 208, no. 3, pp. 973–986. https://doi.org/10.1111/nph.13496
Gomory, D., Paule, L., and Mačejovsky, V., Phylogeny of beech in Western Eurasia as inferred by approximate Bayesian computation, Acta Soc. Bot. Pol., 2018, vol. 87, no. 2, p. 3582. https://doi.org/10.5586/asbp.3582
Semerikov, N.V., Petrova, I.V., Sannikov, S.N., et al., Cytoplasmic DNA variation does not support a recent contribution of Pinus sylvestris L. from the Caucasus to the main range, Tree Genet. Genomes, 2020, vol. 16, no. 4, p. 59. https://doi.org/10.1007/s11295-020-01458-8
Semerikova, S.A., Chloroplast DNA markers on the phylogeography study of roburoid oaks (Quercus L. sect. Quercus, Fagaceae) in the Crimean-Caucasian region, Genetika, 2023, vol. 59, no. 1, pp. 50–64. https://doi.org/10.31857/S0016675823010095
Opredelitel’ vysshikh rastenii Kryma (Key for Higher Plants of Crimea), Rubtsov, N.I., Ed., Leningrad: Nauka, 1972.
Devey, M.E., Bell, J.C., Smith, D.N., et al., A genetic linkage map for Pinus radiata based on RFLP, RAPD and microsatellite markers, Theor. Appl. Genet., 1996, vol. 92, no. 6, pp. 673–679. https://doi.org/10.1007/BF00226088
Deguilloux, M.F., Dumolin-Lapegue, S., Gielly, L., et al., A set of primers for the amplification of chloroplast microsatellites in Quercus, Mol. Ecol. Notes, 2003, vol. 3, no. 1, pp. 24–27. https://doi.org/10.1046/j.1471-8286.2003.00339.x
Hall, T.A., BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT, Nucleic Acids Symp. Ser., 1999, vol. 41, pp. 95–98.
Excoffier, L. and Lischer, H., ARLEQUIN Ver. 3.5: An Integrated Software Package for Population Genetics Data Analysis, Bern: Univ. Bern, Inst. Ecol. Evol., Comp. Mol. Popul. Genet. Lab. (CMPG), 2011.
Nei, M., Molecular Evolutionary Genetics, New York: Columbia Univ., 1987.
Ronquist, F. and Huelsenbeck, J.P., MrBAYES 3: Bayesian phylogenetic inference under mixed models, Bioinformatics, 2003, vol. 19, no. 12, pp. 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Swofford, D.L., PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0 Beta10, Sunderland: Sinauer Assoc., 2002.
Semerikova, S.A., Isakov, I.Yu, and Semerikov, V.L., Chloroplast DNA variation shed light on the history of lime tree (Tilia cordata s. l.) in the eastern part of the range, Russ. J. Genet., 2020, vol. 56, no. 2, pp. 192–203. https://doi.org/10.1134/s1022795420020118
Ingvarsson, P.K., Ribstein, S., and Taylor, D.R., Molecular evolution of insertions and deletion in the chloroplast genome of Silene, Mol. Biol. Evol., 2003, vol. 20, no. 11, pp. 1737–1740. doi https://doi.org/10.1093/molbev/msg163
Bandelt, H.J., Forster, P., and Röhl, A., Median-joining networks for inferring intraspecific phylogenies, Mol. Biol. Evol., 1999, vol. 16, no. 1, pp. 37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Quercus Portal. https://quercusportal.pierroton.inra.fr/index.php?p=GENOMIC_SEQ
Pham, K.K., Hipp, A.L., Manos, P.S., and Cronn, R.C., A time and a place for everything: Phylogenetic history and geography as joint predictors of oak plastome phylogeny, Genome, 2017, vol. 60, no. 9, pp. 720–732. https://doi.org/10.1139/gen-2016-0191
Hipp, A.L., Manos, P.S., Hahn, M., et al., Genomic landscape of the global oak phylogeny, New Phytol., 2020, vol. 226, no. 4, pp. 1198–1212. https://doi.org/10.1111/nph.16162
Curtu, A.L., Sofletea, N., Toader, A.V., and Enescu, M.C., Leaf morphological and genetic differentiation between Quercus robur L. and its closest relative, the drought-tolerant Quercus pedunculiflora K. Koch., Ann. For. Sci., 2011, vol. 68, no. 7, pp. 1163–1172. https://doi.org/10.1007/s13595-011-0105-z
Atanassova, A., Palaeoecological setting of the western Black Sea area during the last 15000 years, Holocene, 2005, vol. 15, pp. 576–584. https://doi.org/10.1191/0959683605hl832rp
Ferris, C., King, R.A., Vainola, R., and Hewitt, G.M., Chloroplast DNA recognises three refugial sources of European oaks and shows independent eastern and western immigrations to Finland, Heredity, 1998, vol. 80, pp. 584–593.
Jensen, J.S., Gillies, A., Csaikl, U., et al., Chloroplast DNA variation within the Nordic countries, For. Ecol. Manage., 2002, vol. 156, pp. 167–180. https://doi.org/10.1016/S0378-1127(01)00641-7
Gomory, D., Paule, L., Krajmerova, D., et al., Admixture of genetic lineages of different glacial origin: A case study of Abies alba Mill. in the Carpathians, Plant Syst. Evol., 2012, vol. 298, pp. 703–712. https://doi.org/10.1007/s00606-011-0580-6
Bolikhovskaya, N.S., Porotov, A.V., Richards, K., et al., Detailed reconstructions of Holocene climate and environmental changes in the Taman Peninsula (Kuban River delta region) and their correlation with rapid sea-level fluctuations of the Black Sea, Quat. Int., 2018, vol. 465, pp. 22–36. https://doi.org/10.1016/j.quaint.2017.08.013
ACKNOWLEDGMENTS
The authors are grateful to L.I. Agafonov, N.V. Semerikov, E.G. Filippov, V.V. Kukarskikh, Kh.U. Aliev, T.V. Semerikova, M.A. Polezhaeva, E.S. Kashirina, V.V. Korzhenevsky for help in collecting oak samples, and to an anonymous reviewer for constructive comments.
Funding
The study was supported by the Russian Science Foundation, grant no. 22-24-00667. https://rscf.ru/en/project/22-24-00667/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of the welfare of humans or animals. The article does not contain any studies involving humans or animals in experiments performed by any of the authors.
Supplementary Information
Rights and permissions
About this article
Cite this article
Semerikova, S.A., Podergina, S.M., Tashev, A.N. et al. Phylogeography of Oaks in the Crimea Reveals Pleistocene Refugia and Migration Routes. Russ J Ecol 54, 197–212 (2023). https://doi.org/10.1134/S1067413623030049
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1067413623030049