Abstract
• Introduction
The study of phenotypic and genetic differentiation between incipient species or species that have recently diverged provides insights into the evolutionary history of species complexes and may contribute to our understanding of how species will evolve in contrasting environmental conditions.
• Objective
Here, we characterise the differences in leaf morphology and we estimate the genetic differentiation between Quercus robur and its closest relative, the drought-tolerant Quercus pedunculiflora. We have examined whether these two ecologically divergent taxa have different genetic structures using both nuclear and chloroplast markers.
• Results
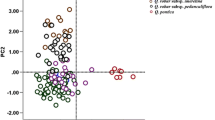
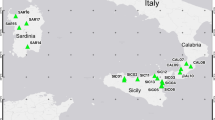
By analysing 844 individual trees from seven Q. robur and seven Q. pedunculiflora populations and one mixed forest containing both taxa, we found that abaxial laminar pubescence is the most discriminating leaf descriptor between Q. robur and Q. pedunculiflora. The analysis of seven enzyme-coding gene loci revealed no taxon-specific alleles with a frequency > 0.03. The DNA chloroplast haplotypes observed in Q. pedunculiflora have been found in our Q. robur sample or have been previously reported in Q. robur.
• Conclusions
The very low level of nuclear divergence revealed by the isozyme markers and the incomplete sorting of Q. robur and Q. pedunculiflora populations according to their physical appearance suggests that Q. pedunculiflora is an incipient oak species and that the process of ecological speciation is not completed.



Similar content being viewed by others
References
Arnold M, Martin N (2009) Adaptation by introgression. J Biol 8:82
Belletti P, Leonardi S et al (2005) Allozyme variation in different species of deciduous oaks from northwestern Italy. Silvae Genetica 54:9–16
Bordács S, Popescu F et al (2002) Chloroplast DNA variation of white oaks in the northern Balkans and in the Carpathian Basin. For Ecol Manage 156:197–209
Broshtilov K (2006) Quercus robur L. leaf variability in Bulgaria. Plant Genetic Resources Newsletter 147:64–71
Bussotti F (1998) Q. pubescens Willd. In: Schütt P, Schuck HJ, Lang UM and Roloff A (Eds), Enzyklopädie der Holzgewächse: Handbuch und Atlas der Dendrologie, Ecomed, Landsberg am Lech, pp 1–10
Chesnoiu EN, Sofletea N et al (2009) Bud burst and flowering phenology in a mixed oak forest from Eastern Romania. Ann For Res 52:199–206
Ciocarlan V (2000) Flora ilustrata a Romaniei. Editura Ceres, Bucuresti, 1139 p
Curtu AL, Gailing O et al (2007) Genetic variation and differentiation within a natural community of five oak species (Quercus spp). Plant Biology 9:116–126
De Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56:879–886
Donita N, Geambasu T et al (2004) Dendrologie. Editura Vasile Goldis, Arad, 423 p
Donita N, Popescu A et al (2005) Habitatele din Romania. Editura Tehnica Silvica, Bucuresti, 496 p
Enescu V (1993) A test of half-sib progenies of greyisch oak. Quercus pedunculiflora K Koch. Ann Sci For 50:439–443
Excoffier L, Laval G et al (2005) Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evolutionary Bioinforma Online 1:47–50
Finkeldey R (2001) Genetic variation of oaks (Quercus spp.) in Switzerland. 1. Allelic diversity and differentiation at isozyme gene loci. For Genet 8:185–195
Fortini P, Viscosi V et al (2009) Comparative leaf surface morphology and molecular data of five oaks of the subgenus Quercus Oerst (Fagaceae). Plant Biosyst 143:543–554
Georgescu CC, Cretzoiu P (1941) Consideratiuni sistematice asupra speciei Quercus pedunculiflora K Koch. in Romania. Analele ICAS 7:3–37
Georgescu CC, Morariu J (1948) Monografia stejarilor din România. Universul, Bucuresti, 26 p
Gömöry D, Schmidtova J (2007) Extent of nuclear genome sharing among white oak species (Quercus L. subgen. Lepidobalanus (Endl.) Oerst.) in Slovakia estimated by allozymes. Plant Syst Evol 266:253–264
Gömöry D, Yakovlev I et al (2001) Genetic differentiation of oak populations within the Quercus robur/Quercus petraea complex in Central and Eastern Europe. Heredity 86:557–563
Gömöry D, Longauer R et al (2010) Across-species patterns of genetic variation in forest trees of Central Europe. Biodivers Conserv 19:2025–2038
Hey J (2006) Recent advances in assessing gene flow between diverging populations and species. Curr Opin in Genet & Development Genomes and evolution 16:592–596
Kremer A (2007) How well can existing forests withstand climate change. In: Koskela J, Buck A, Teissier du Cros E (eds) Climate change and forest genetic diversity: implications for sustainable forest management in Europe. Bioversity International, Rome, pp 3–17
Kremer A, Dupouey JL et al (2002) Leaf morphological differentiation between Quercus robur and Quercus petraea is stable across western European mixed oak stands. Ann For Sci 59:777–787
Lepais O, Petit RJ et al (2009) Species relative abundance and direction of introgression in oaks. Mol Ecol 18:2228–2242
Lexer C, Kremer A et al (2006) Shared alleles in sympatric oaks: recurrent gene flow is a more parsimonious explanation than ancestral polymorphism. Mol Ecol 15:2007–2012
Menitsky YL (2005) Oaks of Asia, Science Publishers, p 549
Muir G, Fleming CC et al (2000) Species status of hybridizing oaks. Nature 405:1016
Müller-Starck G, Zanetto A et al (1996) Inheritance of isoenzymes in sessile oak (Quercus petraea (Matt.) Liebl.) and offspring from interspecific crosses. For Genet 3:1–12
Nei M, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6:288–295
Petit RJ, Excoffier L (2009) Gene flow and species delimitation. Trends in Ecology and Evolution 24:386–393
Petit R, Brewer S et al (2002a) Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For Ecol Manage 156:49–74
Petit R, Csaikl U et al (2002b) Chloroplast DNA variation in European white oaks. Phylogeography and patterns of diversity based on data from over 2600 populations. For Ecol Manage 156:5–26
Pons O, Petit RJ (1995) Estimation, variance and optimal sampling of gene diversity. I. Haploid locus. Theor Appl Genet 90:462–470
Potenko V, Koren O et al (2007) Genetic variation and differentiation in populations of Japanese emperor oak Quercus dentata Thunb. and Mongolian oak Quercus mongolica Fisch. ex Ledeb. in the south of the Russian Far East. Russ J Genet 43:387–395
Regent Instruments I (2007) WinFOLIA: Image analysis systems and software, Regent Instruments Inc
Rushton BS (1993) Natural hybridization within the genus Quercus. Ann Sci For 50:73–90
Schwarz O (1937) Monographie der Eichen Europas und des Mittelmeergebietes I. Textband, Dahlem, Berlin, 200 p
Schwarz O (1993) Quercus L. In: Tutin TG, Burges NA, Chater AO (eds) Flora Europaea. Cambridge University Press, Cambridge, pp 72–76
Stanescu V, Sofletea N et al (1997) Flora forestiera lemnoasa a Romaniei. Editura Ceres, Bucuresti
StatSoft (2008) STATISTICA for Windows [Software-System For Data Analysis]
Tamura K, Dudley J et al (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24:1596–1599
Toader A, Moldovan IC et al (2009) DNA isolation and amplification in oak species (Quercus spp.). Bulletin of the Transilvania University of Brasov 2 Series II: 45–50
Turner TL, Hahn MW (2007) Locus- and population-specific selection and differentiation between incipient species of Anopheles gambiae. Mol Biol Evol 24:2132–2138
Viscosi V, Lepais O et al (2009) Leaf morphological analyses in four European oak species Quercus and their hybrids: A comparison of traditional and geometric morphometric methods. Plant Biosystems 143:564–574
Zanetto A, Roussel G et al (1994) Geographic variation of inter-specific differentiation between Quercus robur L. and Quercus petraea (Matt.) Liebl. For Genet 1:111–123
Zanetto A, Kremer A et al (1996) Inheritance of isozymes in pedunculate oak (Quercus robur L.). J Hered 87:364–370
Acknowledgements
We are indebted to numerous colleagues from the forest districts across the country for assisting us during the field sampling. We are grateful to Ecaterina Chesnoiu and Andras Tothpal for field assistance and help in the leaf measurements and Tudor Stancioiu for his suggestions on an earlier version of the manuscript. We wish to also thank two anonymous reviewers for constructive comments on the manuscript. This work was funded by CNCSIS–UEFISCSU, project number PNII-IDEI 183/2007. Mihai Cristian Enescu acknowledges a PhD scholarship (POSDRU/88/1.5/S/59321) financed by ESF and the Romanian Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Luc Paques
Rights and permissions
About this article
Cite this article
Curtu, A.L., Sofletea, N., Toader, A.V. et al. Leaf morphological and genetic differentiation between Quercus robur L. and its closest relative, the drought-tolerant Quercus pedunculiflora K. Koch. Annals of Forest Science 68, 1163–1172 (2011). https://doi.org/10.1007/s13595-011-0105-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13595-011-0105-z