Abstract
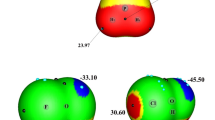
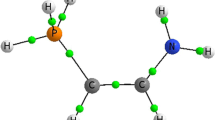
Electron attachment to 1-chloronaphthalene molecules is studied with the aid of the dissociative electron attachment spectroscopy. It is shown that the dominant channel for the decay of molecular ions is the formation of Clˉ ions in three resonances at 0.7, 1.5, and 3.0 eV. The [M–H]ˉ and [M–Cl]ˉ ions are observed at energies from 3.5 to 8.5 eV and exhibit formation cross sections that are less by two-to-three orders of magnitude. Long-lived molecular ions are not detected. The calculations in the DFT CAM B3LYP/6-311+G(d,p) approximation predict the presence of six stable anionic structures in which the chlorine anion is coordinated with the neutral residue via noncovalent H–Clˉ–H bonds. The electron affinity of the most stable of these structures coincides with the experimental value EAa = 0.2771 ± 0.003 eV. Such results are in agreement with the existing data on the dissociative electron attachment to molecules of bromine-substituted biphenyls, naphthalenes, and anthracenes and proves the existence of anionic structures with non-covalent H–Hal–H bonds. Such non-covalent anion structures must be extremely reactive, which makes them promising for the synthesis of self-assembling hydrocarbon nanomembranes.





Similar content being viewed by others
REFERENCES
V. I. Khvostenko, Negative Ions Mass Spectrometry in Organic Chemistry (Nauka, Moscow, 1981) [in Russian].
E. Illenberger and B. M. Smirnov, Phys.-Usp. 41 (7), 651 (1998).
S. A. Pshenichnyuk, N. L. Asfandiarov, A. S. Vorob’ev, and Š. Matejčík, Phys.-Usp. 65 (2), 163 (2022). https://doi.org/10.3367/UFNe.2021.09.039054
N. L. Asfandiarov, M. V. Muftakhov, S. A. Pshenichnyuk, P. Papp, M. Danko, M. Lacko, J. Blaško, Š. Matejčik, and A. Modelli, J. Chem. Phys. 147, 234302 (2017).
N. L. Asfandiarov, M. V. Muftakhov, S. A. Pshenichnyuk, R. G. Rakhmeev, A. M. Safronov, A. V. Markova, A. S. Vorob’ev, T. F. M. Luxford, J. Kočišek, and J. Fedor, J. Chem. Phys. 155, 244302 (2021). https://doi.org/10.1063/5.0074013
N. Takeda, P. V. Poliakov, A. R. Cook, and J. R. Miller, J. Am. Chem. Soc. 126 (13), 4301 (2004).
N. L. Asfandiarov, S. A. Pshenichnyuk, R. G. Rakhmeyev, R. F. Tuktarov, N. L. Zaitsev, A. S. Vorob’ev, J. Kočišek, J. Fedor, and A. Modelli, J. Chem. Phys. 150, 114304 (2019). https://doi.org/10.1063/1.5082611
N. L. Asfandiarov, M. V. Muftakhov, R. G. Rakhmeev, A. M. Safronov, A. V. Markova, and S. A. Pshenichnyuk, J. Electron. Spectrosc. Relat. Phenom. 256, 147178 (2022).
P. Longevialle, Mass Spectrom. Rev. 11 (3), 157 (1992).
A. G. Suits, Annu. Rev. Phys. Chem. 71, 77 (2020).
N. L. Asfandiarov, S. A. Pshenichnyuk, A. S. Vorob’ev, E. P. Nafikova, Y. N. Elkin, D. N. Pelageev, E. A. Koltsova, and A. Modelli, Rapid Commun. Mass Spectrom. 28 (14), 1580 (2014). https://doi.org/10.1002/rcm.6934
A. A. Makarov, A. L. Malinovsky, and E. A. Ryabov, Phys.-Usp. 55 (10), 977 (2012). https://doi.org/10.3367/UFNe.0182.201210e.1047
N. L. Asfandiarov, S. A. Pshenichnyuk, A. S. Vorob’ev, E. P. Nafikova, and A. Modelli, Rapid Commun. Mass Spectrom. 29 (9), 910 (2015). https://doi.org/10.1002/rcm.7162
E. S. Chen and E. C. M. Chen, Rapid Commun. Mass Spectrom. 32 (7), 604 (2018). https://doi.org/10.1002/rcm.8072
J. C. Steelhammer and W. E. Wentworth, J. Chem. Phys. 51 (5), 1802 (1969). https://doi.org/10.1063/1.1672262
S. A. Pshenichnyuk, A. S. Vorob’ev, and A. Modelli, J. Chem. Phys. 135 (18), 184301 (2011). https://doi.org/10.1063/1.3658372
A. M. Scheer and P. D. Burrow, J. Phys. Chem. B 110 (36), 17751 (2006).
A. Modelli, Phys. Chem. Chem. Phys. 5 (14), 2923 (2003).
T. Koopmans, Physica 1 (1–6), 104 (1934).
P. D. Burrow, G. A. Gallup, and A. Modelli, J. Phys Chem. A 112, 4106 (2008).
S. Koch, C. D. Kaiser, P. Penner, M. Barclay, L. Frommeyer, D. Emmrich, P. Stohmann, T. Abu-Husein, A. Terfort, D. H. Fairbrother, O. Ingolfsson, and A. Golzhauser, Beilstein J. Nanotechnol. 8, 2562 (2017).
M. Cipriani, R. Bjornsson, M. Barclay, A. Terfort, D. H. Fairbrother, and O. Ingolfsson, Int. J. Mass Spectrom. 459, 116452 (2021).
Funding
This work was supported by the Russian Science Foundation (project no. 19-13-00021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Chikishev
Rights and permissions
About this article
Cite this article
Asfandiarov, N.L., Muftakhov, M.V., Safronov, A.M. et al. Non-Covalent Structures of Negative Ions Formed upon Dissociative Electron Attachment to Molecules. Tech. Phys. 67, 563–569 (2022). https://doi.org/10.1134/S1063784222080023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784222080023