Abstract
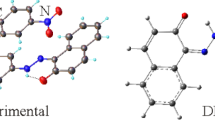
The title compound, C13H14BrN3O2, was synthesized by click chemistry (CuAAC) using 4-bromobut-1-yne and ethyl 4-azidobenzoate. Its molecular structure was determined by 1H NMR, 13C NMR, IR spectra, elemental analysis, and the crystal structure was determined by single crystal X-ray diffraction analysis. It belongs to monoclinic system: space group P21/c, a = 4.9556(6) Å, b = 10.4549(7) Å, c = 27.417(2) Å, β = 93.442(6)°, Z = 4, and V = 1417.9(2) Å3. In the crystal structure, intermolecular hydrogen bonds C–HTrz⋅⋅⋅NTrz and C–HBrethy⋅⋅⋅BrBrethy (Trz = triazole and Brethy = bromoethyl) link the molecules into infinite chains along the a-axis; in this direction, they can be effective in stabilizing the structure. Weak C–H⋅⋅⋅π interaction is also observed. Hirshfeld surface analysis of the crystal structure indicates that the most important contributions to the crystal packing are made by the interactions H…H (37.8%), H…Br/Br…H (17.8%), H…N/N…H (14.9%), H…C/C…H (11.0%), and H…O/O…H (10.8%). Hydrogen bonds and van der Waals interactions are the dominant interactions in crystal packing.








Similar content being viewed by others
REFERENCES
S. Ahmad, O. Alam, M. J. Naim, et al., Eur. J. Med. Chem. 157, 527 (2018).
K. Bozorov, H. R. Ma, J. Y. Zhao, et al., Eur. J. Med. Chem. 84, 739 (2014).
K. Bozorov, L. F. Nie, J. Zhao, and H. A. Aisa, Eur. J. Med. Chem. 140, 465 (2017).
H. Khanam, Eur. J. Med. Chem. 97, 483 (2015).
R. Naik, D. S. Harmalkar, X. Xu, et al., Eur. J. Med. Chem. 90, 379 (2015).
A. Ayati, S. Emami, and A. Foroumadi, Eur. J. Med. Chem. 109, 380 (2016).
K. R. Abdellatif and R. B. Bakr, Bioorg. Chem. 78, 341 (2018).
I. Briguglio, S. Piras, P. Corona, et al., Eur. J. Med. Chem. 97, 612 (2015).
L. Dymińska, Bioorgan. Med. Chem. 23, 6087 (2015).
P. Thirumurugan, D. Matosiuk, and K. Jozwiak, Chem. Rev. 113, 4905 (2013).
S. G. Agalave, S. R. Maujan, and V. S. Pore, Chem-Asıan J. 6, 2696 (2011).
R. M. Meudtner, M. Ostermeier, R. Goddard, et al., Chem-Eur. J. 13, 9834 (2007).
Rigaku Oxford Diffraction, CrysAlis PRO Software System (Rigaku Corporation, Wroclaw, Poland, 2015).
G. M. Sheldrick, Acta Crystallogr. A 64, 112 (2008).
L. J. Farrugia, J. Appl. Crystallogr. 45, 849 (2012).
L. J. Farrugia, J. Appl. Crystallogr. 30, 565 (1997).
A. L. Spek, Acta Crystallogr. D 65, 148 (2009).
L. J. Farrugia, J. Appl. Crystallogr. 32, 837 (1999).
C. P. Kaushik, K. Kumar, S. Singh, et al., Arab. J. Chem. 9, 865 (2016).
T. T. Lan, D. T. Anh, E. J. Park, et al., Med. Chem. Res. 29, 396 (2020).
R. K. Tittal, V. D. Ghule, N. Kumar, et al., J. Mol. Struct. 1209, 127951 (2020).
R. González-Olvera, A. Espinoza-Vázquez, G. E. Negrón-Silva, et al., Molecules 18, 15064 (2013).
P. Yadav, K. Lal, L. Kumar, et al., Eur. J. Med. Chem. 155, 263 (2018).
C. Travelli, S. Aprile, R. Rahimian, et al., J. Med. Chem. 60, 1768 (2017).
F. L. Hirshfeld, Theor. Chim. Acta 44, 129 (1977).
M. A. Spackman and D. Jayatilaka, CrystEngComm 11, 19 (2009).
M. J. Turner, J. J. McKinnon, S. K. Wolff, et al., CrystalExplorer17 (The University of Western Australia, 2017).
P. Venkatesan, S. Thamotharan, A. Ilangovan, et al., Spectrochim. Acta A 153, 625 (2016).
M. A. Spackman, J. J. McKinnon, and D. Jayatilaka, CrystEngComm 10, 377 (2008).
D. Jayatilaka, D. J. Grimwood, A. Lee, et al., TONTO: A System for Computational Chemistry (2005). http://hirshfeldsurface.net/.
J. J. McKinnon, D. Jayatilaka, and M. A. Spackman, Chem. Commun. 37, 3814 (2007).
V. R. Hartwar, M. Sist, M. R. V. Jorgensen, et al., IUCrJ 2, 563 (2015).
A. Taia, M. Essaber, A. Aatif, et al., Acta Crystallogr. E 76, 962 (2020).
N. Abad, Y. Ramli, T. Hökelek, et al., Acta Crystallogr. E 74, 1915 (2018).
J. Zukerman-Schpector, C. da S. Dias, R. S. Schwab, et al., Acta Crystallogr. E 74, 1195 (2018).
J. Zukerman-Schpector, S. D. Pedroso, L. S. Madureira, et al., Acta Crystallogr. E 73, 1716 (2017).
N. Pokhodylo, Y. Slyvkab, and V. Pavlyuk, Acta Crystallogr. E 76, 756 (2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Göktürk, T., Hökelek, T. & Güp, R. Synthesis, Crystal Structure and Hirshfeld Surface Analysis of Ethyl 4-(4-(2-Bromoethyl)-1H-1,2,3-triazol-1-yl)benzoate. Crystallogr. Rep. 66, 977–984 (2021). https://doi.org/10.1134/S1063774521060109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774521060109