Abstract
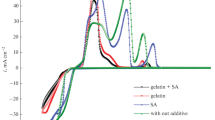
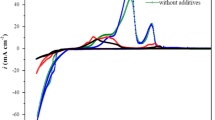
In this report, the mechanism of electrodeposition of Zn–Fe–Co alloy on a steel surface at different deposition time and concentrations of cobalt ions was precisely investigated. To clarify the deposition mechanism, various electrochemical methods were applied. In particular, potentiodynamic polarization was used to ascertain the corrosion behavior of the deposited films. The results indicate that the increase of the sweeping rate and the increase of the Co2+ concentrations in the deposition bath leads to a significant improvement for the steel anti-corrosion takes place. The film contents were carefully analyzed by AAS and EDX. Furthermore, the coated films were also characterized by SEM and XRD to identify the surface morphology and structure, respectively. The alloys electrodeposition, under the used experimental conditions, was an anomalous type. The outcomes indicate the following series of events: Co ions adsorbed on the substrate in the first few seconds; followed by adsorption of Fe ions and then Zn ions onto the freshly adsorbed and deposited Co, means that the normal codeposition. With time progressing, the adsorption of zinc ions suppresses the subsequent accumulation of iron and cobalt, although it does not completely block it.












Similar content being viewed by others
REFERENCES
Liu, Z., Cui, T., Pulletikurthi, G., Lahiri, A., Carstens, T., and Olschewski, M., Dendrite-free nanocrystalline zinc electrodeposition from an ionic liquid containing nickel triflate for rechargeable Zn-based batteries, Angew. Chem. Int. Ed., 2016, vol. 55, p. 2889. https://doi.org/10.1002/anie.201509364
Hu, C., Xie, X., Zheng, H., Qing, Y., and Ren, K., Facile fabrication of superhydrophobic zinc coatings with corrosion resistance via an electrodeposition process, New J. Chem., 2020, vol. 44, p. 8890. https://doi.org/10.1039/D0NJ00561D
Conrad, H.A., McGuire, M.R., Zhou, T., Coskun, M.I., and Golden, T.D., Improved corrosion resistant properties of electrochemically deposited zinc–nickel alloys utilizing a borate electrolytic alkaline solution, J. Coat. Technol. Res., 2015, vol. 272, p. 50. https://doi.org/10.1016/j.surfcoat.2015.04.025
Assaf, F.H., Eissa, A.A., and Abou-Krisha, M.M., Electrodeposition mechanism of Zn‒Ni‒Mn alloy at different time intervals, Russ. J. Appl. Chem., 2018, vol. 91, p. 510. https://doi.org/10.1134/S1070427218030254
Wykpis, K., Niedbala, J., Popczyk, M., Budniok, A., and Lagiewka, E., The electrodeposition and properties of Zn–Ni + Ni composite coatings, Russ. J. Electrochem., 2012, vol. 48, p. 1123. https://doi.org/10.1134/S1023193512080149
Kasach, A.A., Kharitonov, D.S., Radchenko, S.L., Zharskii, I.M., and Kurilo, I.I., Effect of parameters of pulse electrolysis on electrodeposition of copper–tin alloy from sulfate electrolyte, Russ. J. Electrochem., 2020, vol. 56, p. 744. https://doi.org/10.1134/S1023193520090049
Hussein, R.K., Abou-Krisha, M.M., and Yousef, T.A., Theoretical and experimental studies of different amine compounds as corrosion inhibitors for aluminum in hydrochloric acid, Biointerface Res. Appl. Chem., 2021, vol. 11, p. 9772. https://doi.org/10.33263/BRIAC112.97729785
Assaf, F.H., Abou-Krisha, M.M., Daoush, W.M., and Eissa, A.A., Fabrication of Zn–Ni–Mn alloy by electrodeposition and its characterization, Corros. Rev., 2018, vol. 36, p. 547. https://doi.org/10.1515/corrrev-2018-0003
Mehl, S., Toghan, A., Bauer, T., Brummel, O., Taccardi, N., Wasserscheid, P., and Libuda, J., Pd nanoparticle formation in ionic liquid thin films monitored by in situ vibrational spectroscopy, Langmuir, 2015, vol. 31, p. 12126. https://doi.org/10.1021/acs.langmuir.5b03386
Abou-Krisha, M.M., Rageh, H.M., and Matter, E.A., Electrochemical studies on the electrodeposited Zn–Ni–Co ternary alloy in different media, Surf. Coat. Technol., 2008, vol. 202, p. 3739. https://doi.org/10.1016/j.surfcoat.2008.01.015
Faid, H., Mentar, L., Khelladi, M.R., and Azizi, A., Deposition potential effect on surface properties of Zn–Ni coatings, Surf. Eng., 2017, vol. 33, p. 529. https://doi.org/10.1080/02670844.2017.1287836
Eliaz, N., Venkatakrishna, K., and Chitharanjan, H.A., Electroplating and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co alloys, Surf. Coat. Technol., 2010, vol. 205, p. 1969. https://doi.org/10.1016/j.surfcoat.2010.08.077
Abou-Krisha, M.M., Assaf, F.H., Alduaij, O.K., and Eissa, A.A., Deposition potential influence on the electrodeposition of Zn–Ni–Mn alloy, Trans. Indian Inst. Met., 2017, vol. 70, p. 31. https://doi.org/10.1007/s12666-016-0859-y
Assaf, F.H., Abou-Krisha, M.M., Yousef, T.A., Abushoffa, A.M., El-Sheref, F., and Toghan, A., Influence of current density on the mechanism of electrodeposition and dissolution of Zn–Fe–Co alloys, Russ. J. Phys. Chem. A, 2020, vol. 94, p. 1708. https://doi.org/10.1134/S0036024420080026
Abou-Krisha, M.M., Assaf, F.H., and El-Naby, S.A., The influence of Fe2+ concentration and deposition time on the corrosion resistance of the electrodeposited zinc–nickel–iron alloys, Arab. J. Chem., 2016, vol. 9, p. S1349. https://doi.org/10.1016/j.arabjc.2015.10.008
Bhat, R. and Hegde, A.C., Studies electrodeposited Zn–Fe alloy coating on mild steel and its characterization, J. Electrochem. Sci. Eng., 2019, vol. 9, p. 9. https://doi.org/10.5599/jese.565
Abou-Krisha, M.M., Influence of Ni2+ concentration and deposition potential on the characterization of thin electrodeposited Zn–Ni–Co coatings, Mater. Chem. Phys., 2011, vol. 125, p. 621. https://doi.org/10.1016/j.matchemphys.2010.10.007
Abou-Krisha, M.M., Assaf, F.H., and Toghan, A.A., Electrodeposition of Zn–Ni alloys from sulfate bath, J. Solid State Electrochem., 2007, vol. 11, p. 244. https://doi.org/10.1007/s10008-006-0099-x
Abou-Krisha, M.M., Assaf, F.H., and El-Naby, S.A., Electrodeposition behavior of zinc–nickel–iron alloys, from sulfate bath, J. Coat. Technol. Res., 2009, vol. 6, p. 391. https://doi.org/10.1007/s11998-008-9134-4
Brenner, A., Electrodeposition of Alloys, New York: Acad. Press, 1963, vol. 2, p. 194.
Dahms, H. and Croll, I.M., The anomalous codeposition of nickel–iron alloys, J. Electrochem. Soc., 1965, vol. 112, p. 771. https://doi.org/10.1149/1.2423692
Yunus, M., Capel-Boute, C., and Decroly, C., Inhibition effect of zinc on the cathodic deposition of cobalt-I. Electrochemical and structural observations in sulphate solutions, Electrochim. Acta, 1965, vol. 10, p. 885. https://doi.org/10.1016/0013-4686(65)80001-9
Mindowicz, J., Capel-Boute, C., and Decroly, C., Inhibition effect of zinc on the cathodic deposition of cobalt-II. Potentiodynamic and intensiodynamic curves in chloride solutions, Electrochim. Acta, 1965, vol. 10, p. 901. https://doi.org/10.1016/0013-4686(65)80002-0
Lana, C.J., Liua, W.Y., Kea, S.T., and Chin, T.S., Potassium salt based alkaline bath for deposition of Zn–Fe alloys, J. Coat. Technol. Res., 2006, vol. 201, p. 3103. https://doi.org/10.1016/j.surfcoat.2006.06.027
Hosseini, M.G., Ashassi-Sorkhabi, H., and Ghiasvand, H.A.Y., Electrochemical studies of Zn–Ni alloy coatings from non-cyanide alkaline bath containing tartrate as complexing agent, J. Coat. Technol. Res., 2008, vol. 202, p. 2897. https://doi.org/10.1016/j.surfcoat.2007.10.022
Akiyama, T. and Fukushima, H., Recent study on the mechanism of the electrodeposition of iron-group metal alloys, ISIJ Int., 1992, vol. 32, p. 787. https://doi.org/10.2355/isijinternational.32.787
Fukushima, H., Akiyama, T., Yano, M., Ishikawa, T., and Kammel, R., Electrodeposition behavior of Zn–iron-group metal alloys from sulfate and chloride baths, ISIJ Int., 1993, vol. 33, p. 1009. https://doi.org/10.2355/isijinternational.33.1009
Tsuru, T., Kobayashi, S., Akiyama, T., Fukushima, H., Gogia, S.K., and Kammel, R., Electrodeposition behaviour of zinc–iron group metal alloys from a methanol bath, J. Appl. Electrochem., 1997, vol. 27, p. 209. https://doi.org/10.1023/A:1018460109175
Charoen-Amornkitt, P., Suzuki, T., and Tsushima, S., Ohmic resistance and constant phase element effects on cyclic voltammograms using a combined model of mass transport and equivalent circuits, Electrochim. Acta, 2017, vol. 258, p. 433. https://doi.org/10.1016/j.electacta.2017.11.079
Díaz-Arista, P., Meas, Y., Ortega, R., and Trejo, G., Electrochemical and AFM study of Zn electrodeposition in the presence of benzylideneacetone in a chloride-based acidic bath, J. Appl. Electrochem., 2005, vol. 35, p. 217. https://doi.org/10.1007/s10800-004-6304-7
Haque, F., Rahman, M., Ahmed, E., Bakshi, P., and Shaikh, A., A cyclic voltammetric study of the redox reaction of Cu(II) in presence of ascorbic acid in different pH media, Dhaka Univ. J. Sci., 2013, vol. 61, p. 161. https://doi.org/10.3329/dujs.v61i2.17064
El Fazazi, A., Ouakki, M., and Cherkaoui, M., Electrochemical deposition of zinc on mild steel, Mediterr. J. Chem., 2019, vol. 8, p. 30. https://doi.org/10.13171/mjc8119021318mo
Gomez, E., Liorente, A., and Valles, E., Obtention and characterisation of cobalt+copper electrodeposits from a citrate bath, J. Electroanal. Chem., 2000, vol. 495, p. 19. https://doi.org/10.1016/S0022-0728(00)00376-4
Correia, A.N. and Machado, S.A.S., Anodic linear sweep voltammetric analysis of Ni–Co alloys electrodeposited from dilute sulfate baths, J. Appl. Electrochem., 2003, vol. 33, p. 367. https://doi.org/10.1023/A:1024457930014
Gomez, E., Alcobe, X., and Valles, E., Characterisation of zinc+cobalt alloy phases obtained by electrodeposition, J. Electroanal. Chem., 2001, vol. 505, p. 54. https://doi.org/10.1016/S0022-0728(01)00450-8
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mortaga Abou-Krisha, Toghan, A., Assaf, F. et al. The Mechanism and Corrosion Behavior of Zn–Fe–Co Film Electrochemically Deposited on a Steel Substrate: Influence of Deposition Time and Co Ion Concentration. Russ J Electrochem 58, 284–295 (2022). https://doi.org/10.1134/S1023193522040036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522040036