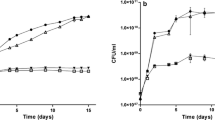
Abstract
Phototrophic non-sulfur bacteria (PNSB) are close relatives of cytokinin producing agrobacteria. However, the sequenced PNSB genomes do not have the ipt gene sequences involved in the cytokinin biosynthesis. In Rhodobacter sphaeroides and Rhodopseudomonas palustris transformed with ipt, we previously observed the substantial morphological and physiological changes as compared with the original strains. To find associations of these changes with the ipt function, its expression levels were studied in the transformants and the parent strains. Unexpectedly, the ipt transcripts were found in both the transformants and the original untransformed strains, although the ipt expression level was several orders of magnitude lower in the latter compared to the transformants. The ipt sequences amplified from the plasmid DNA templates of the parent PNSB strains showed that they were identical to each other but differed in four nucleotides compared to the ipt gene from the Agrobacterium tumefaciens pTiBo 542 plasmid. The in silico comparison amongst the ipt sequences from the other agrobacterial plasmids, as well as from the other PNSB, demonstrated their low identity. This is the first report on the active gene of the cytokinin biosynthesis found in Rba. sphaeroides and Rps. palustris which is homologous to the agrobacterial ipt gene.
Similar content being viewed by others
References
Kakimoto, T., Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases, Plant Cell Physiol., 2001, vol. 42, pp. 677–685. doi 10.1093/pcp/pce112
Sakakibara, H., Cytokinins: activity, biosynthesis, and translocation, Annu. Rev. Plant Biol., 2006, vol. 57, pp. 431–449. doi 0.1146/annurev.arplant.57.032905.105231
Yevdakova, N.A. and von Schwartzenberg, K., Characterisation of a prokaryote-type tRNA-isopentenyltransferase gene from the moss Physcomitrella patens, Planta, 2007, vol. 226, pp. 683–695. doi 10.1007/s00425-007-0516-0
Golovko, A., Hjalm, G., Sitbon, F., and Nicander, V., Cloning of human tRNA isopentenyltransferase, Gene, 2000, vol. 258, pp. 85–93. http://dx.doi.org/. doi 10.1016/S0378-1119(00)00421-2
Romanov, G.A., How do cytokinins affect the cell?, Russ. J. Plant. Physiol., 2009, vol. 56, no. 2, pp. 268–290. https://doi.org/10.1134/S1021443709020174.
Hrtyan, M., Šliková, E., Hejátko, J., and Ružicka, K., RNA processing in auxin and cytokinin pathways, J. Exp. Bot., 2015, vol. 66, pp. 4897–4912. doi 10.1093/jxb/erv189
Lutova, L.A., Genetic plant engineering: achievements and hopes, Sorosovsky Obrazovatelny Zh., 2000, vol. 6, pp. 10–17.
An, G., Binary Ti plasmid vectors, Methods Mol. Biol., 1995, vol. 44, pp. 47–58. doi 10.1385/0-89603-302-3:47
Alekseeva, V.V., Rukavtsova, E.B., Shutova, T.V., et al., Physiological and biochemical traits of tobacco plants carrying an agrobacterial isopentenyltransferase gene, Russ. J. Plant. Physiol., 2000, vol. 47, pp. 408–415.
Strabala, T.J., Bednarek, S.Y., Bertoni, G., and Amasino, R.M., Isolation and characterization of an ipt gene from the Ti plasmid Bo542, Mol. Gen. Genet., 1989, vol. 216, pp. 388–394.
Serdyuk, O.P., Smolygina, L.D., Chekunova, E.M., et al., Direct transition of pGA482:ipt plasmid bearing the cytokinin biosynthesis gene into the cells of phototrophic purple bacteria Rhodobacter sphaeroides and Rhodopseudomonas palustris by electroporation, Dokl. Biochem. Biophys., 2013, vol. 451, nos. 1-6, pp. 194–197. doi 10.1134/S1607672913040078
Serdyuk, O.P., Shirshikova, G.N., Smolygina, L.D., et al., Phenotypic and physiological changes in the phototrophic purple nonsulfur bacterium Rhodopseudomonas palustris transformed with the binary vector pGA482 carrying the cytokinin biosynthesis gene ipt, Dokl. Biochem. Biophys., 2007, vol. 415, nos. 1–6, pp. 191–193. doi 10.1134/S1607672907040072
Machulin, A.V., Smolygina, L.D., Suzina, N.E., and Serdyuk, O.P., Study of phototrophic purple bacterium Rhodobacter sphaeroides cell morphology of wild-type and ipt-transformant by atomic force and electron microscopy, Biophysics, 2012, vol. 57, no. 1, pp. 72–75. doi 10.1134/S0006350912010149
Serdyuk, O., Smolygina, L., Kobsar, E.F., and Gogotov, I.N., Occurence of plant hormones in cells of phototrophic purple bacterium Rhodospirillum rubrum 1R, FEMS Microbiol. Lett., 1993, vol. 109, pp. 113–116.
Woese, C.R., Bacterial evolution, Microbiol. Rev., 1987, vol. 51, no. 2, pp. 221–271. PMID: 2439888
Cohen-Bazire, G., Sistrom, W., and Stainer, R., Kinetic studies of pigment synthesis by non-sulfur purple bacteria, J. Cell Comp. Physiol., 1957, vol. 49, pp. 25–68.
Ormerod, J.S., Ormerod, S.R., and Gest, H., Light dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria: relationships with nitrogen metabolism, Arch. Biochem. Biophys., 1961, vol. 94, pp. 449–463.
Maniatis, T., Fritsch, E.F., and Sambrook, J., Molecular Cloning: A Laboratory Manual, Cold Spring Harbor: Cold Spring Harbor Lab., 1982.
Pfaffl, M.W., A new mathematical model for relative quantification in real-time RT–PCR, Nucleic Acids Res., 2001, vol. 29, pp. 2002–2007. doi 10.1093/nar/29.9.e45
Livak, K.J. and Schmittgen, T.D., Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method, Methods, 2001, vol. 25, pp. 402–408. doi 10.1006/meth.2001.1262
Losurdo, L., Italiano, F., Trotta, M., et al., Assessment of an internal reference gene in Rhodobacter sphaeroides grown under cobalt exposure, J. Basic Microbiol., 2010, vol. 50, pp. 302–305. doi 10.1002/jobm.200900340
Altschul, S.F., Gish, W., Mille, W., et al., Basic local alignment search tool, J. Mol. Biol., 1990, vol. 215, pp. 403–410. doi 10.1016/S0022-2836(05)80360-2
Powell, G.K., Hommes, N.G., Kuo, J., et al., Inducible expression of cytokinin biosynthesis in Agrobacterium tumefaciens by plant phenolics, Mol. Plant—Microbe Interact., 1988, vol. 1, pp. 235–242. PMID: 2980282
Oger, P.M., Farrand, S.K., Olsen, G.J., and Reich, C., Complete sequence of the Ti plasmid pTiBo542 from the supervirulent Agrobacterium tumefaciens strain Bo542. http://www.genoma.jp/dbget-bin/www_bget? refseq:NC_010929.
Heidekamp, F., Dirkse, W.G., Hille, J., and van Ormondt, H., Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene, Nucleic Acids Res., 1983, vol. 11, pp. 6211–6223. PMCID:PMC326368
Akiyoshi, D.E., Regier, D.A., Jen, G., and Gordon, M.P., Cloning and nucleotide sequence of the tzs gene from Agrobacterium tumefaciens strain T37, Nucleic Acids Res., 1985, vol. 13, pp. 2773–2788. PMCID: PMC341193
Yanofsky, M.F., Lowe, B., Montoya, A., Rubin, R., Krul, W., Gordon, M., and Nester, E., Molecular and genetic analysis of factors controlling host range in Agrobacterium tumefaciens, Mol. Gen. Genet., 1985, vol. 201, pp. 237–246.
Serdyuk, O.P., Smolygina, L.D., Muzafarov, E.N., et al., 4-Hydroxyphenethyl alcohol—a new cytokininlike substance from the phototrophyc purple bacterium Rhodospirillum rubrum 1R, FEBS Lett., 1995, vol. 365, pp. 10–12. doi 10.1016/0014-5793(95)00430-H
Serdyuk, O.P., Smolygina, L.D., Ivanova, E.P., et al., 4-Hydroxyphenethyl alcohol—a new cytokinin-like substance from the phototrophyc purple bacterium Rhodospirillum rubrum: exhibition of activity on plants and transformed mammalian cells, Proc. Biochem., 2000, vol. 36, pp. 475–479.
Balakhnina, T., Wlodarczyk, T., Borkowska, A., et al., Effect of 4-hydroxyphenethyl alcohol on growth and adaptive potential of barley plants under optimal and soil flooding conditions, Pol. J. Environ. Stud., 2010, vol. 19, pp. 565–572.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.P. Serdyuk, G.N. Shirshikova, L.D. Smolygina, A.M. Butanaev, V.D. Kreslavsky, N.V. Yartseva, E.M. Chekunova, 2017, published in Genetika, 2017, Vol. 53, No. 10, pp. 1179–1186.
Rights and permissions
About this article
Cite this article
Serdyuk, O.P., Shirshikova, G.N., Smolygina, L.D. et al. Agrobacterial ipt gene for cytokinin biosynthesis is found in phototrophic non-sulfur purple bacteria Rhodobacter sphaeroides and Rhodopseudomonas palustris . Russ J Genet 53, 1113–1118 (2017). https://doi.org/10.1134/S102279541710009X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102279541710009X