Abstract
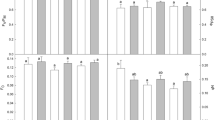
The thermal stability of plants with different types of photosynthesis Chenopodium quinoa Willd. (C3) and Amaranthus retroflexus L. (C4-NAD) to short-term elevated temperature (35°С, eT) at ambient (400 ppm, aCO2) and elevated (800 ppm, eCO2) CO2 concentration was investigated. Growth parameters, water, proline, and MDA content, CO2/H2О gas exchange parameters, the functioning of PS I and PS II, and the content of key photosynthetic (Rubisco, PEPC) and photorespiratory (GDC) enzymes were analyzed. Under control conditions, C4-type plants, compared with the C3-type, show higher values of dry biomass growth, intensity of visible photosynthesis, transpiration, and PS I activity and lower proline content. The photosynthetic and stomatal apparatus of both types was sensitive to eT, which manifested itself in a decrease in the intensity of apparent photosynthesis and transpiration. In addition, suppression of light reactions (PS II) and intensity of photorespiration (according to GDC) was observed in the C3-species and an increase in the content of proline in the C4-species. Under eCO2 conditions, the C3-species showed a decrease in the intensity of photorespiration, while oxidative stress (twofold increase in the content of MDA) was accompanied by reduced intensity of apparent photosynthesis, transpiration, and increased intensity of dark mitochondrial respiration in the C4-species. A softening effect of eCO2 on thermal stability data for C3- and C4-plants was not established. With the combined action of eCO2 and eT, both types exhibited oxidative stress, reduced efficiency of PS II and apparent photosynthesis, and activation of dark respiration. However, differences were also observed: oxidative stress was accompanied by a decrease in the increase in dry biomass and water content in tissues, as well as suppression of photorespiration, in the C3-species, while there was a decrease in the intensity of transpiration and an increase in the content of PEPC in the C4-species. Reduced WUE with combined action of eCO2 and eT in plants of the C4-species was less significant than the C3-species. The different response of quinoa plants (C3) and amaranth (C4) on the combined effect of climatic factors of elevated temperature and CO2 concentration is discussed.





Similar content being viewed by others
REFERENCES
Ain, Q.T., Siddique, K., Bawazeer, S., Ali, I., Mazhar, M., Rasool, R., Mubeen, B., Ullah, F., Unar, A., and Jafar, T.H., Adaptive mechanisms in quinoa for coping in stressful environments: an update, PeerJ., 2023, vol. 11, p. e14832. https://doi.org/10.7717/peerj.14832
Shanker, A.K., Gunnapaneni, D., Bhanu, D., Vanaja, M., Lakshmi, N.J., Yadav, S.K., Prabhakar, M., and Singh, V.K., Elevated CO2 and water stress in combination in plants: Brothers in arms or partners in crime?, Biol., 2022, vol. 11, p. 1330. https://doi.org/10.3390/biology11091330
Cao, Q., Li, G., and Liu, F., Elevated CO2 enhanced water use efficiency of wheat to progressive drought stress but not on maize, Front. Plant Sci., 2022, vol. 13, p. 953712. https://doi.org/10.3389/fpls.2022.953712
Dusenge, M.E., Duarte, A.G., and Way, D.A., Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration, New Phytol., 2019, vol. 221, p. 32. https://doi.org/10.1111/nph.15283
Yadav, S., Elansary, H.O., Mattar, M.A., Elhindi, M.K., Alotaibi, A.M., and Mishra, A., Differential accumulation of metabolites in Suaeda species provides new insights into abiotic stress tolerance in C4-halophytic species in elevated CO2 conditions, Agronomy, 2021, vol. 11, p. 131. https://doi.org/10.3390/agronomy11010131
Xu, Z., Jiang, Y., and Zhou, G., Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants, Front. Plant Sci., 2015, vol. 6, p. 701. https://doi.org/10.3389/fpls.2015.00701
Reich, P.B., Hobbie, S.E., Lee, T.D., and Pastore, M.A., Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment, Science, 2018, vol. 360, p. 317. https://doi.org/10.1126/science.aas9313
Lara, M.V. and Andreo, C.S., C4 plants adaptation to high levels of CO2 and to drought environments, In: Abiotic Stress in Plants-Mechanisms and Adaptations, Shanker, A., Ed., InTech: Hampshire, UK, 2011, p. 415. https://doi.org/10.5772/24936
Zheng, Y., Li, F., Hao, L., Yu, J., Guo, L., Zhou, H., Ma, C., Zhang, X., and Xu, M., Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean, BMC Plant Biol., 2019, vol. 19, p. 255. https://doi.org/10.1186/s12870-019-1788-9
Jothiramshekar, S., Benjamin, J.J., Krishnasamy, R., Pal, A., George, S., Swaminathan, R., and Parida, A., Responses of selected C3 and C4 halophytes to elevated CO2 concentration under salinity, Curr. Sci., 2018, vol. 115, p. 129. https://doi.org/10.18520/cs/v115/i1/129-135
Faria, A.P., Marabesi, M.A., Gaspar, M., and França, M.G., The increase of current atmospheric CO2 and temperature can benefit leaf gas exchanges, carbohydrate content and growth in C4 grass invaders of the Cerrado biome, Plant Physiol. Biochem., 2018, vol. 127, p. 608. https://doi.org/10.1016/j.plaphy.2018.04.042
Wang, M., Xie, B., Fu, Y., Dong, C., Hui, L., Guanghui, L., and Liu, H., Effects of different elevated CO2 concentrations on chlorophyll contents, gas exchange, water use efficiency, and PSII activity on C3 and C4 cereal crops in a closed artificial ecosystem, Photosynth. Res., 2015, vol. 126, p. 351. https://doi.org/10.1007/s11120-015-0134-9
Boretti, A. and Florentine, S., Atmospheric CO2 concentration and other limiting factors in the growth of C3 and C4 plants, Plants, 2019, vol. 8, p. 92. https://doi.org/10.3390/plants8040092
Prasch, C.M. and Sonnewald, U., Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks, Plant Physiol., 2013, vol. 162, p. 1849. https://doi.org/10.1104/pp.113.221044
Yamori, W., Hikosaka, K., and Way, D.A., Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation, Photosynth Res., 2014, vol. 119, p. 101. https://doi.org/10.1007/s11120-013-9874-6
Hinojosa, L., González, J.A., Barrios-Masias, F.H., Fuentes, F., and Murphy, K.M., Quinoa abiotic stress responses: A review, Plants, 2018, vol. 7, p. 106. https://doi.org/10.3390/plants7040106
Li, G., Chen, T., Feng, B., Peng, S., Tao, L., and Fu, G., Respiration, rather than photosynthesis, determines rice yield loss under moderate high-temperature conditions, Front Plant Sci., 2021, vol. 12, p. 678653. https://doi.org/10.3389/fpls.2021.678653
Sage, R.F. and Kubien, D.K., The temperature response of C3 and C4 photosynthesis, Plant, Cell Environ., 2007, vol. 30, p. 1086. https://doi.org/10.1111/j.1365-3040.2007.01682.x
Wang, D., Heckathorn, S.A., Barua, D., Joshi, P., Hamilton, E.W., and Lacroix, J.J., Effects of elevated CO2 on the tolerance of photosynthesis to acute heat stress in C3, C4, and CAM species, Am. J. Bot., 2008, vol. 95, p. 165. https://doi.org/10.3732/ajb.95.2.165
Zinta, G., AbdElgawad, H., Domagalska, M.A., Vergauwen, L., Knapen, D., and Nijs, I., Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels, Glob. Chang. Biol., 2014, vol. 20, p. 3670. https://doi.org/10.1111/gcb.12626
Zinta, G., AbdElgawad, H., Peshev, D., Weedon, J.T., Van den Ende, W., and Nijs, I., Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospheric CO2, J. Exp. Bot., 2018, vol. 69, p. 2159. https://doi.org/10.1093/jxb/ery055
Zhou, R., Yu, X., Wen, J., Jensen, N.B., Dos Santos, T.M., Wu, Z., Rosenqvist, E., and Ottosen, C.O., Interactive effects of elevated CO2 concentration and combined heat and drought stress on tomato photosynthesis, BMC Plant Biol., 2020, vol. 20, p. 260. https://doi.org/10.1186/s12870-020-02457-6
Arrivault, S., Alexandre Moraes, T., Obata, T., Medeiros, D.B., Fernie, A.R., Boulouis, A., Ludwig, M., Lunn, J.E., Borghi, G.L., Schlereth, A., Guenther, M., and Stitt, M., Metabolite profiles reveal interspecific variation in operation of the Calvin-Benson cycle in both C4 and C3 plants, J. Exp. Bot., 2019, vol. 70, p. 1843. https://doi.org/10.1093/jxb/erz051
Yu, J., Li, R., Fan, N., Yang, Z., and Huang, B., Metabolic pathways involved in carbon dioxide enhanced heat tolerance in bermudagrass, Front. Plant Sci., 2017, vol. 8, p. 1506. https://doi.org/10.3389/fpls.2017.01506
Bordignon, L., Faria, A.P., França, M.G.C., and Fernandes, G.W., Osmotic stress at membrane level and photosystem II activity in two C4 plants after growth in elevated CO2 and temperature, Ann. Appl. Biol., 2019, vol. 174, p. 113. https://doi.org/10.1111/aab.12483
Jeong, H.M., Kim, H.R., Hong, S., and You, Y.H., Effects of elevated CO2 concentration and increased temperature on leaf quality responses of rare and endangered plants, J. Ecol. Environ., 2018, vol. 42. https://doi.org/10.1186/s41610-017-0061-0
Kirschbaum, M.U.F. and McMillan, A.M.S., Warming and elevated CO2 have opposing influences on transpiration. Which is more important?, Curr. Forestry Rep., 2018, vol. 4, p. 51. https://doi.org/10.1007/s40725-018-0073-8
Wei, Z., Abdelhakim, L., Fang, L., Peng, X., Liu, J., and Liu, F., Elevated CO2 effect on the response of stomatal control and water use efficiency in amaranth and maize plants to progressive drought stress, Agric. Water Manage., 2022, vol. 266. https://doi.org/10.1016/j.agwat.2022.107609
Schafleitner, R., Lin, Y.-P., Dinssa, F., N’Danikou, S., Finkers, R., Minja, R., Abukutsa-Onyango, M., Nyonje, W., Lin, C.-Y., Wu, T.-H., Sigalla, J.P., van Zonneveld, M., Hsiao, Y.-Y., Kumar, S., Wu, W.-J., et al., The World Vegetable Center Amaranthus germplasm collection: Core collection development and evaluation of agronomic and nutritional traits, Crop Sci., 2022, vol. 62, p. 1173. https://doi.org/10.1002/csc2.20715
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water stress studies, Plant Soil., 1973, vol. 39, p. 205. https://doi.org/10.1007/BF00018060
Heath, R.L. and Packer, L., Photoperoxidation in isolated chloroplasts, Arch. Biochem. Biophys., 1968, vol. 125, p. 180. https://doi.org/10.1016/0003-9861(68)90654-1
Shuyskaya, E., Rakhmankulova, Z., Prokofieva, M., Saidova, L., Toderich, K., and Voronin, P., Intensity and duration of salinity required to form adaptive response in C4 halophyte Kochia prostrata (L.) Shrad., Front. Plant Sci., 2022, vol. 13. https://doi.org/10.3389/fpls.2022.955880
Nakamura, N., Iwano, M., Havaux, M., Yokota, A., and Munekage, Y.N., Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria, New Phytol., 2013, vol. 199, p. 832. https://doi.org/10.1111/nph.12296
Yadav, S. and Mishra, A., Ectopic expression of C4 photosynthetic pathway genes improves carbon assimilation and alleviate stress tolerance for future climate change, Physiol. Mol. Biol. Plants, 2020, vol. 26, p. 195. https://doi.org/10.1007/s12298-019-00751-8
Crafts-Brandner, S.J. and Salvucci, M.E., Sensitivity of photosynthesis in a C4 plant, maize, to heat stress, Plant Physiol., 2002, vol. 129, p. 1773. https://doi.org/10.1104/pp.002170
Heckathorn, S.A., Ryan, S.L., Baylis, J.A., Wang, J.A., Hamilton, E.W., and Cundiff, L., In vivo evidence from an Agrostis stolonifera selection genotype that chloroplast small heat-shock proteins can protect photosystem II during heat stress, Funct. Plant Biol., 2002, vol. 29, p. 933. https://doi.org/10.1071/PP01191
Singh, S.K. and Reddy, V.R., Methods of mesophyll conductance estimation: its impact on key biochemical parameters and photosynthetic limitations in phosphorus stressed soybean across CO2, Physiol. Plant, 2016, vol. 157, p. 234. https://doi.org/10.1111/ppl.12415
Souza, A.P., Gaspar, M., Silva, E.A., Ulian, E.C., Waclawovsky, A.J., Nishiyama, M.Y. Jr., Santos, R.V., Teixeira, M.M., Souza, G.M., and Buckeridge, M.S., Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane, Plant Cell Environ., 2008, vol. 31, p. 1116. https://doi.org/10.1111/j.1365-3040.2008.01822.x
Huang, Y., Fang, R., Li, Y., Liu, X., Wang, G., Yin, K., Jin, J., Herbert, S.J., Warming and elevated CO2 alter the transcriptomic response of maize (Zea mays L.) at the silking stage, Sci. Rep., 2019, vol. 9, p. 17948. https://doi.org/10.1038/s41598-019-54325-5
Funding
The work was performed with the financial support of the Russian Science Foundation (project no. 23-24-00551).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This work does not contain any studies involving humans and animals as subjects of study.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rakhmankulova, Z.F., Shuyskaya, E.V., Prokofieva, M.Y. et al. Effect of Elevated CO2 and Temperature on Plants with Different Type of Photosynthesis: Quinoa (C3) and Amaranth (C4). Russ J Plant Physiol 70, 117 (2023). https://doi.org/10.1134/S1021443723601349
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443723601349