Abstract
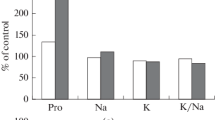
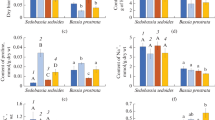
Research into organization and peculiarities of carbon-concentrating mechanism (CCM) functioning in plants with intermediate C3–С4 (C2) photosynthesis type characterized by resistance to arid climate is of paramount importance given the global climate changes. In this study two populations (P1 and P2) of С3–С4 Sedobassia sedoides (Chenopodiaceae) different in productivity were investigated under PEG-induced osmotic stress. As a result of our studies, a less productive P1 population was classified as the Type II of С2 photosynthesis, since there was a relatively low efficiency of cyclic electron transport around PSI, and high glycine decarboxylase (GDC) content. On the contrary, P2 population was more productive and based on type of photosynthesis was classified as С4-like plants. It exhibited a higher efficiency of cyclic electron transport around PSI and lower GDC content. Plants in each population also demonstrated heterogeneity in terms of the degree of dehydration under osmotic stress and fell into two groups: stress-tolerant (ST) and not stress-tolerant (NST) plants. NST plants from both populations showed a significant increase in proline content and a decrease of maximum quantum yield of PSII (Fv/Fm) evidencing a severe osmotic stress. P1 and P2 plants showed differences in mechanisms ensuring drought tolerance, more pronounced in NST plants. NST plants from P1 showed a decrease in K+/Na+ ratio, an increase in cyclic electron flow around PSI and a decrease in efficiency of PSII (due to NPQ), presence of oxidative stress (an increase in catalase and SOD activity), increase in content of C4 CCM enzymes PEPС and NAD-Me. NST plants from P2 retained considerable dry biomass and shoot length (which were formed before stress), demonstrated a decline in Na+ accumulation, an increase in K+/Na+ ratio, and a decrease in activity of cyclic electron flow around PSI and PSII efficiency (due to NPQ), absence of oxidative stress, a decrease in Rubisco content and content of C4 CCM enzymes PEPC and NAD-Me. The complexity of determining the specific mechanisms of biochemical response of plants to stress is discussed, as well as the high stress-induced plasticity of this type of photosynthesis, i.e. the possibility of switch from one type of C3–C4 photosynthesis to another under drought, either towards enhancing С4 characteristics or towards their weakening.






Similar content being viewed by others
REFERENCES
Lundgren, M., C2 photosynthesis: a promising route towards crop improvement? New Phytol., 2020, vol. 228, p. 1734. https://doi.org/10.1111/nph.16494
Cornic, G., Drought stress inhibits photosynthesis by decreasing stomatal aperture—not by affecting ATP synthesis, Trends Plant Sci., 2000, vol. 5, p. 187. https://doi.org/10.1016/S1360-1385(00)01625-3
Lawlor, D.W., Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP, Ann. Bot., 2002, vol. 89, p. 871. https://doi.org/10.1093/aob/mcf110
Dias, M.C. and Brüggemann, W., Differential inhibition of photosynthesis under drought stress in Flaveria species with different degrees of development of the C4 syndrome, Photosynthetica, 2007, vol. 45, p. 75. https://doi.org/10.1007/s11099-007-0012-6
Munekage, Y.N. and Taniguchi, Y.Y., A scheme for C4 evolution derived from a comparative analysis of the closely related C3, C3–C4 intermediate, C4-like, and C4 species in the genus Flaveria, Plant Mol. Biol., 2022: 35119574. https://doi.org/10.1007/s11103-022-01246-z
Sage, R.F., Khoshravesh, R., and Sage, T.L., From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis, J. Exp. Bot., 2014, vol. 65, p. 3341. https://doi.org/10.1093/jxb/eru180
Johnson, J.E., Field, C.B., and Berry, J.A., The limiting factors and regulatory processes that control the environmental responses of C3, C3–C4 intermediate, and C4 photosynthesis, Oecologia, 2021, vol. 197, p. 841. https://doi.org/10.1007/s00442-021-05062-y
Siadjeu, C., Lauterbach, M., and Kadereit, G., Insights into regulation of C2 and C4 photosynthesis in Amaranthaceae/Chenopodiaceae using RNA-Seq, Int. J. Mol. Sci., 2021, vol. 22, p. 12120. https://doi.org/10.3390/ijms222212120
Lundgren, M.R. and Christin, P.A., Despite phylogenetic effects, C3–C4 lineages bridge the ecological gap to C4 photosynthesis, J. Exp. Bot., 2017, vol. 68, p. 241. https://doi.org/10.1093/jxb/erw451
Munekage, Y.N. and Taniguchi, Y.Y., Promotion of cyclic electron transport around photosystem I with the development of C4 photosynthesis, Plant Cell Physiol., 2016, vol. 57, p. 897. https://doi.org/10.1093/pcp/pcw012
Yamori, W. and Shikanai, T., Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth, Annu. Rev. Plant Biol., 2016, vol. 67, p. 81. https://doi.org/10.1146/annurev-arplant-043015-112002
Way, D.A., What lies between: the evolution of stomatal traits on the road to C4 photosynthesis, New Phytol., 2012, vol. 193, p. 291. https://doi.org/10.1111/j.1469-8137.2011.04000.x
Rakhmankulova, Z.F., Photorespiration: Its role in the productive process and evolution of C4 plants, Russ. J. Plant Physiol., 2018, vol. 65, p. 303. https://doi.org/10.1134/S1021443718030068
Sage, R.F., Monson, R.K., Ehleringer, J.R., Adachi, S., and Pearcy, R.W., Some like it hot: the physiological ecology of C4 plant evolution, Oecologia, 2018, vol. 187, p. 941. https://doi.org/10.1007/s00442-018-4191-6
Aubry, S., Kelly, S., Kümpers, B.M.C., Smith-Unna, R.D., and Hibberd, J.M., Deep evolutionary comparison of gene expression identifies parallel recruitment of trans-factors in two independent origins of C4 photosynthesis, PLoS Genet., 2016, vol. 10, p. e1004365. https://doi.org/10.1371/journal.pgen.1004365
Sayre, R.T. and Kennedy, R.A., Ecotypic differences in the C3 and C4 photosynthetic activity in Mollugo verticillata, a C3–C4 intermediate, Planta, 1977. vol. 134, p. 257. https://doi.org/10.1007/BF00384190
Lundgren, M.R., Christin, P.A., Escobar, E.G., Ripley, B.S., Besnard, G., Long, C.M., Hattersley, P.W., Ellis, R.P., Leegood, R.C., and Osborne, C.P., Evolutionary implications of C3–C4 intermediates in the grass Alloteropsis semialata, Plant, Cell Environ., 2016, vol. 39, p. 1874. https://doi.org/10.1111/pce.12665
Rakhmankulova, Z.F., Shuyskaya, E.V., Suyundukov, Ya.T., Usmanov, I.Yu., and Voronin, P.Yu., Different responses of two ecotypes of C3–C4 xero-halophyte Bassia sedoides to osmotic and ionic factors of salt stress, Russ. J. Plant Physiol., 2016, vol. 63, p. 349. https://doi.org/10.1134/S1021443716030122
Shuyskaya, E., Rakhmankulova, Z., Voronin, P., Kuznetsova, N., Biktimerova, G., and Usmanov, I., Salt and osmotic stress tolerance of the C3-C4 xero-halophyte Bassia sedoides from two populations differ in productivity and genetic polymorphism, Acta Physiol. Plant., 2015, vol. 37, p. 236. https://doi.org/10.1007/s11738-015-1981-x
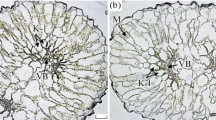
Rakhmankulova, Z.F., Shuyskaya, E.V., Khalilova, L.A., Burundukova, O.L., Velivetskaya, T.A., Ignat’ev, A.V., and Orlova, Yu.V., Ultra- and mesostructural response to salinization in two populations of C3-C4 intermediate species Sedobassia sedoides, Russ. J. Plant Physiol., 2020a, vol. 67, p. 835. https://doi.org/10.1134/S1021443720040135
Rakhmankulova, Z.F., Shuyskaya, E.V., Voronin, P.Yu., Velivetskaya, T.A., Ignatiev, A.V., and Usmanov, I.Yu., Role of photorespiration and cyclic electron transport in C4 photosynthesis evolution in the C3–C4 intermediate species Sedobassia sedoides, Russ. J. Plant Physiol., 2018, vol. 65, p. 455. https://doi.org/10.1134/S102144371802005X
Rakhmankulova, Z.F., Shuyskaya, E.V., Prokofieva, M.Yu., Borovkov, A.M., and Voronin, P.Yu., Comparative contribution of CO2/H2O exchange components to the process of adaptation to drought in xero-halophytes from the family Chenopodiaceae with different types of photosynthesis, Russ. J. Plant Physiol., 2020b, vol. 67, p. 494. https://doi.org/10.1134/S102144372003019X
El Moukhtari, A., Cabassa-Hourton, C., Farissi, M., and Savouré, A., How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci., 2020, vol. 11, p. 1127. https://doi.org/10.3389/fpls.2020.01127
Katschnig, D., Jaarsma, R., Almeida, P., Rozema, J., and Schat, H., Differences in proton pumping and Na/H exchange at the leaf cell tonoplast between a halophyte and a glycophyte, AoB Plants, 2014, vol. 6:plu023. https://doi.org/10.1093/aobpla/plu023
Polesskaya, O.G., Rastitel’naya kletka i aktivnye formy kisloroda (Plant Cell and Reactive Oxygen Species), Moscow: KDU, 2007.
Goncharenko, G.G., Padutov, V.E., and Potenko, V.V., Rukovodstvo po issledovaniyu khvoinykh vidov metodom elektroforeticheskogo analiza izofermentov (Manual for the Study of Coniferous Species by Electrophoretic Analysis of Isoferments), Gomel’: Polespechat’, 1989.
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, p. 205. https://doi.org/10.1007/BF00018060
Klughammer, C. and Schreiber, U., Measuring P700 absorbance changes in the near infrared spectral region with a dual wavelength pulse modulation system, in Photosynthesis: Mechanisms and Effects, Garab, G., Ed., Dordrecht: Kluwer Academic Publishers, 1998, p. 4357. https://doi.org/10.1007/978-94-011-3953-3_1008
Nakamura, N., Iwano, M., Havaux, M., Yokota, A., and Munekage, Y.N., Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria, New Phytol., 2013, vol. 199, p. 832. https://doi.org/10.1111/nph.12296
Schreiber, U., Chlorophyll Fluorescence and Photosynthetic Energy Conversion: Simple Introductory Experiments with the TEACHING-PAM Chlorophyll Fluorometer, Effeltrich, Germany: Heinz Walz, 1997.
Pozhidaeva, E.S., Western blot hybridization, Molekulyarno-geneticheskie i biokhimicheseskie metody v sovremennoi biologii rastenii (Molecular-Genetic and Biochemical Methods in Modern Plant Biology), Kuznetsov, Vl.V., Kuznetsov, V.V., and Romanov, G.A., Eds., Moscow: BINOM, Laboratoriya Znanii, 2011.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, vol. 72, p. 248. https://doi.org/10.1006/abio.1976.9999
Laemmli, U.K., Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, vol. 227, p. 680. https://doi.org/10.1038/227680a0
Beauchamp, C. and Fridovich, I., Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, vol. 44, p. 276. https://doi.org/10.1016/0003-2697(71)90370-8
Aebi, H., Catalase in vitro, Methods Enzymol., 1984, vol. 105, p. 121. https://doi.org/10.1016/s0076-6879(84)05016-3
Guidi, L., Lo Piccolo, E., and Landi, M., Chlorophyll fluorescence, photoinhibition and abiotic stress: does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci., 2019, vol. 10, p. 174. https://doi.org/10.3389/fpls.2019.00174
Brestic, M. and Zivcak, M., PSII fluorescence techniques for measurement of drought and high temperature stress signal in plants: protocols and applications, Molecular Stress Physiology of Plants, Rout, G.R. and Das, A.B., Eds., Dordrecht: Springer, 2013, p. 87. https://doi.org/10.1007/978-81-322-0807-5
Khatri, K. and Rathore, M.S., Salt and osmotic stress-induced changes in physio-chemical responses, PSII photochemistry and chlorophyll a fluorescence in peanut, Plant Stress, 2022, vol. 3, p. 100063. https://doi.org/10.1016/j.stress.2022.100063
Azzabi, G., Pinnola, A., Betterle, N., Bassi, R., and Alboresi, A., Enhancement of non-photochemical quenching in the bryophyte Physcomitrella patens during acclimation to salt and osmotic stress, Plant Cell Physiol., 2012, vol. 53, p. 1815. https://doi.org/10.1093/pcp/pcs124
Holaday, A.S., Lee, K.W., and Chollet, R., C3–C4 intermediate species in the genus Flaveria: leaf anatomy, ultrastructure, and the effect of O2 on the CO2 compensation concentration, Planta, 1984, vol. 160, p. 25. https://doi.org/10.1007/BF00392462
Funding
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme no.: 122042700044-6).
Author information
Authors and Affiliations
Contributions
Authors ZFR and EVS designed the experiments. ZFR, EVS, and MYuP collected samples and performed the experiments. ZFR drafted the manuscript and all authors revised it.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Rights and permissions
About this article
Cite this article
Rakhmankulova, Z.F., Shuyskaya, E.V. & Prokofieva, M.Y. Intraspecific Photosynthetic Diversity and Differences in Stress-Induced Plasticity in С3–С4 Sedobassia sedoides under Drought Stress. Russ J Plant Physiol 70, 81 (2023). https://doi.org/10.1134/S1021443722603135
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722603135