Abstract
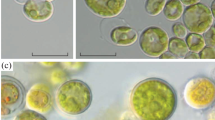
Green microalgae capable of accumulating secondary carotenoids are the most important objects of biotechnology, and the search for new strains with unique properties, in particular, those adapted to growth at low temperatures and high salinity in the environment, is an urgent task. The NAMSU SBB-20 microalga strain was isolated from an algal-bacterial biofilm found on the coast of the White Sea in the littoral zone of the Solovetsky Archipelago. Identification of the strain showed its belonging to the species Halochlorella rubescens P.J.L.Dangeard. The species H. rubescens was first described for the White Sea. Under conditions of high light intensity, ultrastructural changes in cells are shown, among which destruction of the photosynthetic apparatus and the formation of cytoplasmic and chloroplast lipid inclusions are noted. It was shown that the culture of the NAMSU SBB-20 strain is capable of acquiring an orange color under unfavorable growth conditions. An assessment was made of the effect of the composition of the medium and the intensity of illumination on the pigment composition of the algae. The highest absolute values of the accumulation of carotenoids were noted during cultivation in light with an intensity of 150 μmol PAR quanta/m2/s on BG-11 media containing no source of phosphorus (15.66 ± 0.18 mg/L) or nitrogen (15.95 ± 0.56 mg/L). The described strain has a biotechnological potential due to the initial halotolerance and the accumulation of high values of secondary carotenoids in the biomass.





Similar content being viewed by others
REFERENCES
Borowitzka, M.A., Commercial production of microalgae: ponds, tanks, tubes and fermenters, J. Biotechnol., 1999, vol. 70, nos. 1–3, p. 313. https://doi.org/10.1016/S0168-1656(99)00083-8
Guerin, M., Huntley, M., and Olaizola, M., Haematococcus astaxanthin: applications for human health and nutrition, Trends Biotechnol., 2003, vol. 21, no. 5, p. 210. https://doi.org/10.1016/S0167-7799(03)00078-7
Solovchenko, A. and Chekanov, K., Production of carotenoids using microalgae cultivated in photobioreactors, Production of Biomass and Bioactive Compounds Using Bioreactor Technology, Paek, K.Y., Murthy, H., and Zhong, J., Eds., Dordrecht: Springer, 2014, p. 63. https://doi.org/10.1007/978-94-017-9223-3_4
Chekanov, K., Fedorenko, T., Kublanovskaya, A., Litvinov, D., and Lobakova, E., Diversity of carotenogenic microalgae in the White Sea polar region, FEMS Microbiol. Ecol., 2020, vol. 96, p. 183. https://doi.org/10.1093/femsec/fiz183/5632105
Stanier, R., Kunisawa, R., Mandel, M., and Cohen-Bazire, G., Purification and properties of unicellular blue-green algae (order Chroococcales), Bacteriol. Rev., 1971, vol. 35, p. 171. https://doi.org/10.1128/br.35.2.171-205.1971
Temraleeva, A.D., Mincheva, E.V., Bukin, Yu.S., and Andreeva, A.M., Sovremennye metody vydeleniya, kul’tivirovaniya i identifikatsii zelenykh vodorosley (Chlorophyta) (Modern Methods of Isolation, Cultivation and Identification of Green Algae (Chlorophyta)), Kostroma: Kostromskoy pechatnyy dom, 2014.
Wang, Y., Tian, R.M., Gao, Z.M., Bougouffa, S., and Qian, P.Y., Optimal eukaryotic 18S and universal 16S/18S ribosomal RNA primers and their application in a study of symbiosis, PloS One, 2014, vol. 9, no. 3, p. e90053. https://doi.org/10.1371/journal.pone.0090053
Ismagulova, T., Chekanov, K., Gorelova, O., Baulina, O., Semenova, L., Selyakh, I., Chivkunova, O., Lobakova, E., Karpova, O., and Solovchenko, A., A new subarctic strain of Tetradesmus obliquus—part I: identification and fatty acid profiling, J. Appl. Phycol., 2018, vol. 30, no. 5, p. 2737. https://doi.org/10.1007/s10811-017-1313-1
Maltsev, Y., Gusev, E., Maltseva, I., Kulikovskiy, M., Namsaraev, Z., Petrushkina, M., Filimonova, A., Sorokin, B., Golubeva, A., Butaeva, G., Khrushchev, A., Zotko, N., and Kuzmin, D., Description of a new species of soil algae, Parietochloris grandis sp. nov., and study of its fatty acid profiles under different culturing conditions, Algal Res., 2018, vol. 33, p. 358. https://doi.org/10.1016/j.algal.2018.06.008
Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J., Gapped BLAST and PSI-BLAST: a new generation of protein database search programs, Nucleic Acids Res., 1997, vol. 25, no. 17, p. 3389. https://doi.org/10.1093/nar/25.17.3389
Edgar, G.J., Stuart-Smith, R.D., Willis, T.J., Kininmonth, S., Baker, S.C., Banks, S., and Thomson, R.J., Global conservation outcomes depend on marine protected areas with five key features, Nature, 2014, vol. 506, no. 7487, p. 216.
Kumar, S., Stecher, G., and Tamura, K., MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets, Mol. Biol. Evol., 2016, vol. 33, no. 7, p. 1870. https://doi.org/10.1093/molbev/msw054
Aldrich, J., RA Fisher and the making of maximum likelihood 1912-1922, Stat Sci., 1997, vol. 12, no. 3, p. 162. https://doi.org/10.1214/ss/1030037906
Kimura, M., A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences, J. Mol. Evol., 1980, vol. 16, no. 2, p. 111. https://doi.org/10.1007/BF01731581
Nei, M. and Kumar, S., Molecular Evolution and Phylogenetics, Oxford: Oxford University Press, 2000.
Felsenstein, J., Confidence limits on phylogenies: an approach using the bootstrap, Evolution, 1985, vol. 39, no. 4, p. 783. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Gorelova, O., Baulina, O., Solovchenko, A., Selyakh, I., Chivkunova, O., Semenova, L., Scherbakov, P., Burakova, O., and Lobakova, E., Coordinated rearrangements of assimilatory and storage cell compartments in a nitrogen-starving symbiotic chlorophyte cultivated under high light, Arch. Microbiol., 2015, vol. 197, p. 181. https://doi.org/10.1007/s00203-014-1036-5
Reynolds, E., The use of lead citrate at high pH as an electron-opaque stain in electron microscopy, J. Cell Biol., 1963, vol. 17, no. 1, p. 208. https://doi.org/10.1083/jcb.17.1.208
Anderson, T.F., Techniques for the preservaation of three-dimensional structure in preparing specimens for the electron microscope, Trans. N.Y. Acad. Sci., 1951, vol. 13, no. 4, p. 130. https://doi.org/10.1111/j.2164-0947.1951.tb01007.x
Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M., and Stanier, R.Y., Generic assignments, strain histories and properties of pure cultures of cyanobacteria, Microbiology, 1979, vol. 111, no. 1, p. 1. https://doi.org/10.1099/00221287-111-1-1
Solovchenko, A., Merzlyak, M.N., Khozin-Goldberg, I., Cohen, Z., and Boussiba, S., Coordinated carotenoid and lipid syntheses induced in parietochloris incisa (Chlorophyta, trebouxiophyceae) mutant deficient in δ5 desaturase by nitrogen starvation and high light, J. Phycol., 2010, vol. 46, no. 4, p. 763. https://doi.org/10.1111/j.1529-8817.2010.00849.x
Guiry, M.D. and Guiry, G.M., AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. https://www.algaebase.org. Accessed November 26, 2022.
Kessler, E., Schäfer, M., Hümmer, C., Kloboucek, A., and Huss, V.A.R., Physiological, biochemical, and molecular characters for taxonomy of the subgenera of Scenedesmus (Chlorococcales, Chlorophyta), Bot. Acta, 1997, vol. 110, p. 244. https://doi.org/10.1111/j.1438-8677.1997.tb00636.x
Kessler, E., Czygan, F.C., Fott, B., and Nováková, M., Über Halochlorella rubescens Dangeard, Protistenk, 1968, vol. 110, p. 462.
Kalina, T. and Puncochárová, M., Taxonomy of the subfamily Scotiellocystoideae Fott 1976 (Chlorellaceae, Chlorophyceae), Archiv für Hydrobiologie. Supplementband. Monographische Beiträge, 1987, vol. 73, no. 4, p. 473.
Huss, V.A., Frank, C., Hartmann, E.C., Hirmer, M., Kloboucek, A., Seidel, B.M., Wenzeler, P., and Kessler, E., Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta), J. Phycol., 1999, vol. 35, no. 3, p. 587. https://doi.org/10.1046/j.1529-8817.1999.3530587.x
Chesunov, A.V., Kalyakina, N.M., and Bubnova, E.N., Katalog bioty Belomorskoy biologicheskoy stantsii MGU (Biota Catalog of the White Sea Biological Station of Moscow State University), Moscow: Tov. Nauch. Izd. KMK, 2008.
Shi, J., Podola, B., and Melkonian, M., Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae, Biores. Technol., 2014, vol. 154, p. 260. https://doi.org/10.1016/j.biortech.2013.11.100
Jo, S.W., Hong, J.W., Do, J.M., Na, H., Kim, J.J., Park, S.I., Kim, Y.S., Kim, I.S., and Yoon, H.S., Nitrogen deficiency-dependent abiotic stress enhances carotenoid production in indigenous green microalga Scenedesmus rubescens KNUA042, for use as a potential resource of high value products, Sustainability, 2020, vol. 12, no. 13, p. 5445. https://doi.org/10.3390/su12135445
Tsavatopoulou, V.D., Aravantinou, A.F., Vakros, J., and Manariotis, I.D., Conversion of Scenedesmus rubescens lipid into biodiesel by biochar of different origin, Catalysts, 2021, vol. 11, no. 9, p. 1116. https://doi.org/10.3390/catal11091116
Zaytseva, A., Chekanov, K., Zaytsev, P., Bakhareva, D., Gorelova, O., Kochkin, D., and Lobakova, E., Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation, Plants, 2021, vol. 10, no. 12, p. 2601. https://doi.org/10.3390/plants10122601
Solovchenko, A.E., Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses, Russ. J. Plant Physiol., 2012, vol. 59, no. 2, p. 167. https://doi.org/10.1134/S1021443712020161
Davidi, L., Shimoni, E., Khozin-Goldberg, I., Zamir, A., and Pick, U., Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil, Plant Physiol., 2014, vol. 164, no. 4, p. 2139. https://doi.org/10.1104/pp.113.235119
Ota, S., Morita, A., Ohnuki, S., Hirata, A., Sekida, S., Okuda, K., Ohya, Y., and Kawano, S., Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis, Sci. Rep., 2018, vol. 8, no. 1, p. 1. https://doi.org/10.1038/s41598-018-23854-w
Chekanov, K., Litvinov, D., Fedorenko, T., Chivkunova, O., and Lobakova, E., Combined production of astaxanthin and β-carotene in a new strain of the microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) cultivated in photobioreactor, Biology, 2021, vol. 10, no. 7, p. 643. https://doi.org/10.3390/biology10070643
ACKNOWLEDGMENTS
We are grateful to Professor of the Department of Bioengineering, Faculty of Biology, Moscow State University, Dr.Bio.Sci. A.E. Solovchenko for help in interpreting some of the results. Electron microscopic studies were carried out using the equipment of the Center for Collective Use of Moscow State University.
Funding
This work was supported by a grant from the president of the Russian Federation (no. MK-1952.2021.1.4) as well as by the Molecular Technologies of Living Systems and Synthetic Biology Scientific and Educational School of Moscow State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies involving humans and animals as subjects. The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zaitseva, A.A., Bakhareva, D.A., Zaitsev, P.A. et al. Characteristics of a New Halotolerant Arctic Strain of Carotenogenic Microalga Halochlorella rubescens NAMSU SBB-20. Russ J Plant Physiol 70, 49 (2023). https://doi.org/10.1134/S1021443722603123
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722603123