Abstract
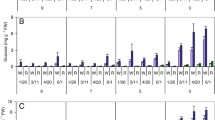
The functional parameters of mitochondria (intactness, oxidation rates of succinate and NAD·H, respiratory control coefficient, ADP/O ratio, and protein content) were studied in freshly harvested and cold-stored tubers of potato Solanum tuberosum L. cv. Skarb, transformed with a gene gox encoding the glucose oxidase from Penicillium funiculosum. Using untransformed potatoes and potatoes transformed with a vector without the target gene and vector constructs carrying the native and modified gox gene, it was shown that its expression causes changes in the rates of oxidation of the studied substrates and the degree of coupling of oxidation and phosphorylation, reduces the potential activity of the alternative oxidase, and induces the synthesis of a number of protective mitochondrial proteins involved in the regulation of the formation of ROS during storage in the cold. The role of hydrogen peroxide in the mechanisms of growth and stress resistance of plants, and its participation in the respiratory metabolism of plant cells is discussed.




Similar content being viewed by others
REFERENCES
Schertl, P. and Braun, H.P., Respiratory electron transfer pathways in plant mitochondria, Front. Plant Sci., 2014, vol. 5, p. 163. https://doi.org/10.3389/fpls.2014.00163
Rasmusson, A., Soole, K., Elthon, T., Alternative NAD(P)H dehydrogenases of plant mitochondria, Annu. Rev. Plant Biol., 2004, vol. 55, p. 23.
Vanlerberghe, G.C., Dahal, K., Alber, N.A., Chadee, A., Photosynthesis, respiration and growth: A carbon and energy balancing act for alternative oxidase, Mitochondrion, 2020, vol. 52, p. 197. https://doi.org/10.1016/j.mito.2020.04.001
Garmash, E.V., Signal pathways for regulation of plant alternative oxidase genes’ expression, Rus. J. Plant Physiol., 2022, vol. 69, p. 1. https://doi.org/10.1134/S1021443722010058
Kesseler, A., Diolez, P., Brinkmann, K., and Brand, M.D., Characterisation of the control of respiration in potato tuber mitochondria using the top-down approach of metabolic control analysis, Eur. J. Biochem., 1992, vol. 210, p. 775.
Shugaev, A.G. and Sokolova, S.V., The changes in the pathways of mitochondrial oxidation at the initial period of tuber development, Rus. J. Plant Physiol., 2001, vol. 48, p. 45.
Shugaev, A.G., Shugaeva, N.A., and Vyskrebentseva, E.I., Cyanide-and rotenone-resistant respiration in mitochondria of sugar beet taproots during plant growth and development, Rus. J. Plant Physiol., 2006, vol. 53, p. 449. https://doi.org/10.1134/S1021443706040030
Golovko T.K. Dykhanie rastenii (fiziologicheskie aspekty) (Plant respiration (physiological aspects)), St. Petersburg: Nauka, 1999, 204 p.
Markarov, A.M., Golovko, T.K., and Tabalenkova, G.N., Morphophysiology of tuber-forming plants, St. Petersburg: Nauka, 2001, 208 p.
Semikhatova, O.A. and Chirkova, T.V., Physiology of plant respiration, St. Petersburg: Publishing House of St. Petersburg University, 2001, 224 p.
Pinheiro, H.A., Borges, R., Silva, M.A.P., and Centeno, D.C., Activity of alternative oxidase and plant uncoupling mitochondrial protein in potato tubers stored at low temperature or submitted to artificial aging, Braz. J. Plant Physiol., 2004, vol. 16, p. 69. https://doi.org/10.1590/S1677-04202004000200001
Mariano, A.B., Valente, C., Cadena, S.M.S.C., Rocha, M.E.M., de Oliveira, M.B.M., and Carnieri, E.G.S., Sensitivities of the alternative respiratory components of potato tuber mitochondria to thiol reagents and Ca2+, Plant Physiol. Biochem., 2005, vol. 43, p. 61. https://doi.org/10.1016/j.plaphy.2004.12.008
Gureev, A.P., Sitnikov, V.V., Pogorelov, D.I., Vitkalova, I.Y., Igamberdiev, A.U., and Popov, V.N., The effect of pesticides on the NADH-supported mitochondrial respiration of permeabilized potato mitochondria, Pestic. Biochem. Physiol., 2022, vol. 183, p. 105056. https://doi.org/10.1016/j.pestbp.2022.105056
Elhafez, D., Murcha, M.W., Clifton, R., Soole, K.L., Day, D.A., and Whelan, J., Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: intraorganelle location and expression, Plant Cell Physiol., 2006, vol. 47, p. 43. https://doi.org/10.1093/pcp/pci221
Grabelnykh, O.I., Pobezhimova, T.P., Pavlovskaya, N.S., Koroleva, N.A., Borovik, O.A., Lyubushkina, I.V., and Voinikov, V.K., Antioxidant function of alternative oxidase in mitochondria of winter wheat during cold hardening, Biochem. (Moscow) Suppl. Series A: Membrane and Cell Biology, 2011, vol. 5, p. 249. https://doi.org/10.1134/S1990747811040040
Considine, M.J., Goodman, M., Echtay, K.S., Laloi, M., Whelan, J., Brand, M.D., Sweetlove, L.J., Superoxide stimulates a proton leak in potato mitochondria that is related to the activity of uncoupling protein, J. Biol. Chem., 2003, vol. 278, p. 22298. https://doi.org/10.1074/jbc.M301075200
Gray, G.R., Maxwell, D.P., Villarimo, A.R., McIntosh, L., Mitochondria/nuclear signaling of alternative oxidase gene expression occurs through distinct pathways involving organic acids and reactive oxygen species, Plant Cell Reports, 2004, vol. 23, p. 497. https://doi.org/10.1007/s00299-004-0848-1
Clifton, R., Lister, R., Parker, K.L., Sappl, P.G., Elhafez, D., Millar, A.H., Day, D.A., and Whelan, J., Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana, Plant Mol. Biol., 2005, vol. 58, p. 193, https://doi.org/10.1007/s11103-005-5514-7
Calegario, F.F., Cosso, R.G., Fagian, M.M., Almeida, F.V., Jardim, W.F., Ježek, P., Arruda, P., and Vercesi, A.E., Stimulation of potato tuber respiration by cold stress is associated with an increased capacity of both plant uncoupling mitochondrial protein (PUMP) and alternative oxidase, J. Bioenerg. Biomembr., 2003, vol. 35, p. 211.
Khan, T., Yusuf, M., and Fariduddin, Q., Hydrogen peroxide in regulation of plant metabolism: Signalling and its effect under abiotic stress, Photosynthetica, 2018, vol. 56, p. 1237. https://doi.org/10.1007/s11099-018-0830-8
Kuźniak, E. and Urbanek, H., The involvement of hydrogen peroxide in plant responses to stresses, Acta Physiol. Plant, 2000, vol. 22, p. 195.
Savchin, D.V., Panyush, A.S., Kartel, N.A., Genetic transformation of plants with vector constructs with the gox Penicillium funiculosum genome, in: Molecular and Applied Genetics: Sat. scientific tr., In. genetics and cytology of the National Academy of Sciences of Belarus; Minsk: Law and Economics, 2011, vol. 12, p. 49.
Savchin, D.V., Veresova, T.N., Mezhnina, O.A., Panyush, A.S., Vyacheslavova, A.O., Goldenkova-Pavlova, I.V., Optimization of the codon composition of the Penicillium funiculosum fungal gox gene for efficient expression in Solanum tuberosum plants, Vesti of the National Academy of Sciences of Belarus Ser. biol. nauk, 2015, vol 1, p. 50.
Grabelnych, O.I., Borovik, O.A., Lyubushkina, I.V., Gamburg, K.Z., Fedyaeva, A.V., Fedoseeva, I.V., Stepanov, A.V., Rikhvanov, E.G., Sauchyn, D.V., Urbanovich, O.Yu., and Borovskii, G.B., Biological effects of potato plants transformation with glucose oxidase gene and their resistance to hyperthermia, J. Stress Physiol. Biochem., 2017, vol. 13, p. 5.
Gamburg, K.Z., Grabelnykh, O.I., Borovik, O.A., and Borovskii, G.B., Influence of the inclusion of the gox gene from Penicillium funiculosum into the genome of the Skarb potato variety on its resistance to long-term cooling, Collection of materials of the Annual Meeting of the Society of Plant Physiologists of Russia, the All-Russian Scientific Conference with International Participation and the School of Young Scientists “Mechanisms of Resistance of Plants and Microorganisms to Unfavorable Environmental Conditions”, Irkutsk: Publishing House of the V.B. Sochavy Institute of Geography of the SB RAS, 2018, vol. II, p. 898.
Maruthasalam, S., Liu, Y.L., Sun, C.M., Chen, P.Y., Yu, C.W., Lee, P.F., and Lin, C.H., Constitutive expression of a fungal glucose oxidase gene in transgenic tobacco confers chilling tolerance through the activation of antioxidative defence system, Plant Cell Rep., 2010, vol. 29, p. 1035. https://doi.org/10.1007/s00299-010-0889-6
Wu, G., Shortt, B.J., Lawrence, E.B., Levine, E.B., Fitzsimmons, K.C., and Shah, D.M., Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants, Plant Cell, 1995, vol. 7, p. 1357.
Bajji, M., M’Hamdi, M., Gastiny, F., Rojas-Beltran, J.A., and du Jardin, P., Catalase inhibition accelerates dormancy release and sprouting in potato (Solanum tuberosum L.) tubers, Biotechnol. Agron. Soc. Environ., 2007, vol. 11, p. 121.
Bellincampi, D., Dipierro, N., Salvi, G., Cervone, F., De Lorenzo, G., Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants, Plant Physiol., 2000, vol. 122, p. 1379.
Neuburger, M., Journet, E.P., Bligny, R., Carde, J.P., Douce, R., Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll, Arch. Biochem. Biophys., 1982, vol. 217, p. 312. https://doi.org/10.1016/0003-9861(82)90507-0
Klimenko, E.S., Study of the features of the import of DNA fragments of different lengths into the mitochondria of Solanum tuberosum, Cand. Sci. (Biol.) Dissertation, Irkutsk: SIPPB SB RAS, 2017, 133 p.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, p. 248.
Pobezhimova, T.P., Korsukova, A.V., Borovik, O.A., Zabanova, N.S., Dorofeev, N.V., Grabelnykh, O.I., Voinikov, V.K., Effect of tebuconazole and tebuconazole-containing preparation “Bunker” on the functioning of winter wheat mitochondria, Biol. Membranes, 2020, vol. 37, p. 224. https://doi.org/10.31857/S0233475520020103
Laemmli, U.K., Cleavage of structural proteins during the assembly of head bacteriophage T4, Nature, 1970, vol. 227, p. 680.
Peters, K., Nießen, M., Peterhänsel, Ch., Späth, B., Hölzle, A., Binder, S., Marchfelder, A., and Braun, H.P., Complex I–complex II ratio strongly differs in various organs of Arabidopsis thaliana, Plant Mol. Biol., 2012, vol. 79, p. 273. https://doi.org/10.1007/s11103-012-9911-4
Svensson, A.S. and Rasmusson, A.G., Light-dependent gene expression for proteins in the respiratory chain of potato leaves, Plant J., 2001, vol. 28, p. 73.
Armstrong, A.F., Murray, R.B., Day, D.A., Barthet, M.M., Smith, P.M.C., Millar, A.H., Whelan, J., and Atkin, O.K., Dynamic changes in the mitochondrial electron transport chain underpinning cold acclimation of leaf respiration, Plant Cell Environ., 2008, vol. 31, p. 1156. https://doi.org/10.1111/j.1365-3040.2008.01830.x
Yosuke, M., Yaptenco, K.F., Tomohiro, N., Toshiro, S., Hiroaki, S., Shinji, M. and Katsumi, T., Property changes in potato tubers (Solanum tuberosum L.) during cold storage at 0 and 10°C, Food Preserv. Sci., 2000, vol. 26, p. 153.
Sweetman, C., Waterman, C.D., Rainbird, B.M., Smith, P.M.C., Jenkins, C.D., Day, D.A., and Soole, K.L., AtNDB2 is the main external NADH dehydrogenase in mitochondria and is important for tolerance to environmental stress, Plant Physiol., 2019, vol. 181, p. 774. https://doi.org/10.1104/pp.19.00877
ACKNOWLEDGMENTS
We thank Olga Yurievna Urbanovich for providing potato lines, Kim Zakharovich Gamburg for introduction of test-tube plants into culture and consultations, and Ekaterina Sergeevna Klimenko for consultations on the isolation of mitochondria from potato tubers. We are also grateful to the anonymous reviewer for valuable remarks and useful advice. The work was carried out using the collection of The Core Facilities Center “Bioresource Center” and the equipment of The Core Facilities Center “Bioanalytics” at The Siberian Institute of Plant Physiology and Biochemistry, Siberian Branch, Russian Academy of Sciences (Irkutsk).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans and animals as research subjects.
Additional information
Translated by M. Shulskaya
Abbreviations: AOX—alternative oxidase; AP—alternative respiration pathway; BHAM—benzhydroxamic acid; HSP101—heat shock protein with a molecular mass of 101 kDa; ND(P)ex (NDB)—“external” rotenone-insensitive NAD(P)·H-dehydrogenases; ND(P)in (NDA)—“internal” rotenone-insensitive NAD(P)·H-dehydrogenases; UCP—uncoupling protein.
Rights and permissions
About this article
Cite this article
Grabelnykh, O.I., Yakovenko, K.V., Polyakova, E.A. et al. Functioning of Mitochondria in Transgenic Potato Tubers with the gox Gene for Glucose Oxidase from Penicillium funiculosum during Different Storage Periods. Russ J Plant Physiol 69, 112 (2022). https://doi.org/10.1134/S1021443722060097
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722060097