Abstract
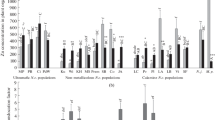
A comparative analysis of nickel (Ni) accumulation by the hyperaccumulator Noccaea japonica (H. Boissieu) F.K. Mey originating from ultramafic (serpentine) soil and plants from 16 populations of the hyperaccumulator Noccaea caerulescens F.K. Mey originating from ultramafic (serpentine), calamine and non-metalliferous soils, was performed. The plants were grown for 2 weeks in half-strength Hoagland’s solution without Ni, followed by a 6-week exposure to NiSO4 at a non-toxic concentration (1 μM). The Ni concentration in the roots and shoots was determined by atomic absorption spectrophotometry. In N. japonica, the Ni concentration in the shoots was significantly lower than in the roots, and lower than that in the shoots of N. caerulescens from the ultramafic populations. The ability of plants from different populations of N. caerulescens to accumulate Ni in roots (per unit dry weight) decreased in the following order: Puente Basadre ≈ Le Coulet > St-Baudille ≈ Cira ≈ Prémanon > Viviez ≈ Monte Prinzera > Les Avinières > Moravskoslezké > Le Bleymard ≈ Krušné Hory ≈ Wilwerwiltz ≈ La Calamine ≈ St-Félix-de-Palliéres ≈ Kuopio > Prayon. The value of the translocation factor in N. japonica did not significantly differ from that in the ultramafic population Puente Basadre of N. caerulescens, whereas it varied within wide limits among the N. caerulescens populations. The highest Ni translocation factor was obtained for the population Monte Prinzera from the ultramafic group and the populations Krušné Hory and Kuopio from the non-metallicolous group, whereas the lowest values were obtained for the calamine populations La Calamine and Prayon. In N. caerulescens, the Ni concentration in the roots was uncorrelated with the Ni concentration in the shoots, but significantly positively correlated with Ni tolerance. The high Ni tolerance in ultramafic populations is apparently explained by a high capacity to sequester Ni in the roots themselves, and not directly related to the root-to-shoot translocation capacity.


Similar content being viewed by others
REFERENCES
Jaffré, T., Pillon, Y., Thomine, S., and Merlot, S., The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants, Front. Plant Sci., 2013, vol. 4, art. ID 279. https://doi.org/10.3389/fpls.2013.00279
van der Ent, A., Vinya, R., Erskine, P.D., Malaisse, F., Przybyłowicz, W.J., Barnabas, A.D., Harris, H.H., and Mesjasz-Przybyłowicz, J., Elemental distribution and chemical speciation of copper and cobalt in three metallophytes from the copper–cobalt belt in Northern Zambia, Metallomics, 2020, vol. 12, p. 682. https://doi.org/10.1039/c9mt00263d
Reeves, R.D., Baker, A.J., Jaffré, T., Erskine, P.D., Echevarria, G., and van der Ent, A., A global database for plants that hyperaccumulate metal and metalloid trace elements, New Phytol., 2018, vol. 218, p. 407. https://doi.org/10.1111/nph.14907
Pollard, A.J., Powell, K.D., Harper, F.A., and Smith, J.A.C., The genetic basis of metal hyperaccumulation in plants, Crit. Rev. Plant. Sci., 2002, vol. 21, p. 539. https://doi.org/10.1080/0735-260291044359
Pollard, A.J., Reeves, R.D., and Baker, A.J.M., Facultative hyperaccumulation of heavy metals and metalloids, Plant Sci., 2014, vol. 217, p. 8. https://doi.org/10.1016/j.plantsci.2013.11.011
Assunção, A.G., Bookum, W.M., Nelissen, H.J., Vooijs, R., Schat, H., and Ernst, W.H., Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types, New Phytol., 2003, vol. 159, p. 411. https://doi.org/10.1046/j.1469-8137.2003.00819.x
Assunção, A.G.L., Bleeker, P., Ten Bookum, W.M., Vooijs, R., and Schat, H., Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary exposures, Plant Soil, 2008, vol. 303, p. 289. https://doi.org/10.1007/s11104-007-9508-x
Richau, K.H., Kozhevnikova, A.D., Seregin, I.V., Vooijs, R., Koevoets, P.L., Smith, J.A., Ivanov, V.B., and Schat, H., Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator Thlaspi caerulescens, New Phytol., 2009, vol. 183, p. 106. https://doi.org/10.1111/j.1469-8137.2009.02826.x
Gonneau, C., Genevois, N., Frérot, H., Sirguey, C., and Sterckeman, T., Variation of trace metal accumulation, major nutrient uptake and growth parameters and their correlations in 22 populations of Noccaea caerulescens, Plant Soil, 2014, vol. 384, p. 271. https://doi.org/10.1007/s11104-014-2208-4
Seregin, I.V., Erlikh, N.T., and Kozhevnikova, A.D., Nickel and zinc accumulation capacities and tolerance to these metals in the excluder Thlaspi arvense and the hyperaccumulator Noccaea caerulescens, Russ. J. Plant Physiol., 2014, vol. 61, p. 204. https://doi.org/10.1134/S1021443714020137
Seregin, I.V., Kozhevnikova, A.D., Zhukovskaya, N.V., and Schat, H., Cadmium tolerance and accumulation in excluder Thlaspi arvense and various accessions of hyperaccumulator Noccaea caerulescens, Russ. J. Plant Physiol., 2015, vol. 62, p. 837. https://doi.org/10.1134/S1021443715050131
Kozhevnikova, A.D., Seregin, I.V., and Schat, H., Accumulation of nickel by excluder Thlaspi arvense and hyperaccumulator Noccaea caerulescens upon short-term and long-term exposure, Russ. J. Plant Physiol., 2020, vol. 67, p. 303. https://doi.org/10.1134/S1021443720020089
Kozhevnikova, A.D., Seregin, I.V., Aarts, M.G., and Schat, H., Intra-specific variation in zinc, cadmium and nickel hypertolerance and hyperaccumulation capacities in Noccaea caerulescens, Plant Soil, 2020, vol. 452, p. 479. https://doi.org/10.1007/s11104-020-04572-7
Seregin, I.V., Kozhevnikova, A.D., and Schat, H., Correlated variation of the Zn accumulation and tolerance capacities among populations and ecotypes of the Zn hyperaccumulator, Noccaea caerulescens, Russ. J. Plant Physiol., 2021, vol. 68, p. S26. https://doi.org/10.1134/S1021443721070128
Sterckeman, T., Cazes, Y., Gonneau, C., and Sirguey, C., Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction, Plant Soil, 2017, vol. 418, p. 523. https://doi.org/10.1007/s11104-017-3311-0
Mizuno, T., Usui, K., Horie, K., Nosaka, S., Mizuno, N., and Obata, H., Cloning of three ZIP/Nramp transporter genes from a Ni hyperaccumulator plant Thlaspi japonicum and their Ni2+-transport abilities, Plant Physiol. Biochem., 2005, vol. 43, p. 793. https://doi.org/10.1016/j.plaphy.2005.07.006
Reeves, R.D., Schwartz, C., Morel, J.L., and Edmondson, J., Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France, Int. J. Phytorem., 2001, vol. 3, p. 145. https://doi.org/10.1080/15226510108500054
Hanikenne, M. and Nouet, C., Metal hyperaccumulation and hypertolerance: a model for plant evolutionary genomics, Curr. Opin. Plant Biol., 2011, vol. 14, p. 252. https://doi.org/10.1016/j.pbi.2011.04.003
Stein, R.J., Höreth, S., de Melo, J.R., Syllwasschy, L., Lee, G., Garbin, M.L., Clemens, S., and Krämer, U., Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri, New Phytol., 2017, vol. 213, p. 1274. https://doi.org/10.1111/nph.14219
Frérot, H., Lefèbvre, C., Petit, C., Collin, C., Dos Santos, A., and Escarré, J., Zinc tolerance and hyperaccumulation in F1 and F2 offspring from intra and interecotype crosses of Thlaspi caerulescens, New Phytol., 2005, vol. 165, p. 111. https://doi.org/10.1111/j.1469-8137.2004.01227.x
Macnair, M.R., Bert, V., Huitson, S.B., Saumitou-Laprade, P., and Petit, D., Zinc tolerance and hyperaccumulation are genetically independent characters, Proc. R. Soc. B, 1999, vol. 266, p. 2175. https://doi.org/10.1098/rspb.1999.0905
Bert, V., Meerts, P., Saumitou-Laprade, P., Salis, P., Gruber, W., and Verbruggen, N., Genetic basis of Cd tolerance and hyperaccumulation in Arabidopsis halleri, Plant Soil, 2003, vol. 249, p. 9. https://doi.org/10.1023/A:1022580325301
Hanikenne, M., Talke, I.N., Haydon, M.J., Lanz, C., Nolte, A., Motte, P., Kroymann, J., Weigel, D., and Krämer, U., Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4, Nature, 2008, vol. 453, p. 391. https://doi.org/10.1038/nature06877
Verbruggen, N., Hermans, C., and Schat, H., Molecular mechanisms of metal hyperaccumulation in plants, New Phytol., 2009, vol. 181, p. 759. https://doi.org/10.1111/j.1469-8137.2008.02748.x
Escarré, J., Lefèbvre, C., Gruber, W., Leblanc, M., Lepart, J., Rivière, Y., and Delay, B., Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implications for phytoremediation, New Phytol., 2000, vol. 145, p. 429. https://doi.org/10.1046/j.1469-8137.2000.00599.x
Seregin, I.V. and Kozhevnikova, A.D., Physiological role of nickel and its toxic effects on higher plant, Russ. J. Plant Physiol., 2006, vol. 53, p. 257. https://doi.org/10.1134/S1021443706020178
Boyd, R.S., The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions, Plant Soil, 2007, vol. 293, p. 153. https://doi.org/10.1007/s11104-007-9240-6
Boyd, R.S., Shaw, J.J., and Martens, S.N., Nickel hyperaccumulation defends Streptanthus polygaloides (Brassicaceae) against pathogens, Am. J. Bot., 1994, vol. 81, p. 294. https://doi.org/10.1002/j.1537-2197.1994.tb15446.x
Kazemi-Dinan, A., Thomaschky, S., Stein, R.J., Krämer, U., and Müller, C., Zinc and cadmium hyperaccumulation act as deterrents towards specialist herbivores and impede the performance of a generalist herbivore, New Phytol., 2014, vol. 202, p. 628. https://doi.org/10.1111/nph.12663
Sharma, S.S., Schat, H., Vooijs, R., and van Heerwaarden, L.M., Combination toxicology of copper, zinc and cadmium in binary mixtures: concentration-dependent antagonistic, non-additive, and synergistic effects on root growth in Silene vulgaris, Environ. Toxicol. Chem., 1999, vol. 18, p. 348. https://doi.org/10.1002/etc.5620180235
Seregin, I.V. and Kozhevnikova, A.D., Low-molecular-weight ligands in plants: role in metal homeostasis and hyperaccumulation, Photosynth. Res., 2021, vol. 150, p. 51. https://doi.org/10.1007/s11120-020-00768-1
Kozhevnikova, A.D., Seregin, I.V., and Schat, H. Translocation of Ni and Zn in Odontarrhena corsica and Noccaea caerulescens: the effects of exogenous histidine and Ni/Zn interactions, Plant Soil, 2021, vol. 468, p. 295. https://doi.org/10.1007/s11104-021-05080-y
Korshunova, Y.O., Eide, D., Clark, W.G., Guerinot, M.L., and Pakrasi, H.B., The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range, Plant Mol. Biol., 1999, vol. 40, p. 37. https://doi.org/10.1023/A:1026438615520
Rogers, E.E., Eide, D.J., and Guerinot, M.L., Altered selectivity in an Arabidopsis metal transporter, Proc. Natl. Acad. Sci. U.S.A., 2000, vol. 97, p. 12356. https://doi.org/10.1073/pnas.210214197
Halimaa, P., Blande, D., Baltzi, E., Aarts, M.G.M., Granlund, L., Keinänen, M., Kärenlampi, S.O., Kozhevnikova, A.D., Peräniemi, S., Schat, H., Seregin, I.V., Tuomainen, M., and Tervahauta, A.L., Transcriptional effects of cadmium on iron homeostasis differ in calamine accessions of Noccaea caerulescens, Plant J., 2019, vol. 97, p. 306. https://doi.org/10.1111/tpj.14121
Nishida, S., Tsuzuki, C., Kato, A., Aisu, A., Yoshida, J., and Mizuno, T., AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana, Plant Cell Physiol., 2011, vol. 52, p. 1433. https://doi.org/10.1093/pcp/pcr089
Nishida, S., Aisu, A., and Mizuno, T., Induction of IRT1 by the nickel-induced iron-deficient response in Arabidopsis, Plant Signaling Behav., 2012, vol. 7, p. 329. https://doi.org/10.4161/psb.19263
Deng, T.H.B., Tang, Y.T., Sterckeman, T., Echevarria, G., Morel, J.L., and Qiu, R.L., Effects of the interactions between nickel and other trace metals on their accumulation in the hyperaccumulator Noccaea caerulescens, Environ. Exp. Bot., 2019, vol. 158, p. 73. https://doi.org/10.1016/j.envexpbot.2018.11.015
Plaza, S., Tearall, K.L., Zhao, F.J., Buchner, P., McGrath, S.P., and Hawkesford, M.J., Expression and functional analysis of metal transporter genes in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens, J. Exp. Bot., 2007, vol. 58, p. 1717. https://doi.org/10.1093/jxb/erm025
Deng, T.H.B., Cloquet, C., Tang, Y.T., Sterckeman, T., Echevarria, G., Estrade, N., Morel, J.-L., and Qiu, R.L., Nickel and zinc isotope fractionation in hyperaccumulating and nonaccumulating plants, Environ. Sci. Technol., 2014, vol. 48, p. 11926. https://doi.org/10.1021/es5020955
Dalir, N., Tandy, S., Gramlich, A., Khoshgoftarmanesh, A., and Schulin, R., Effects of nickel on zinc uptake and translocation in two wheat cultivars differing in zinc efficiency, Environ. Exp. Bot., 2017, vol. 134, p. 96. https://doi.org/10.1016/j.envexpbot.2016.11.009
Craciun, A.R., Meyer, C.L., Chen, J., Roosens, N., De Groodt, R., Hilson, P., and Verbruggen, N., Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation, J. Exp. Bot., 2012, vol. 63, p. 4179. https://doi.org/10.1093/jxb/ers104
Kozhevnikova, A.D., Seregin, I.V., Erlikh, N.T., Shevyreva, T.A., Andreev, I.M., Verweij, R., and Schat, H., Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens, New Phytol., 2014, vol. 203, p. 508. https://doi.org/10.1111/nph.12816
Krämer, U., Metal hyperaccumulation in plants, Ann. Rev. Plant Biol., 2010, vol. 61, p. 517. https://doi.org/10.1146/annurev-arplant-042809-112156
Seregin, I.V. and Kozhevnikova, A.D., Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium, Russ. J. Plant Physiol., 2008, vol. 55, p. 1. https://doi.org/10.1134/S1021443708010019
ACKNOWLEDGMENTS
The authors wish to thank Mark Aarts, Mathilde Mousset, Thibault Sterckeman, Celestino Quintela-Sabarís, Petra Kidd, Oihana Barrutía and Sylvain Merlot for supplying the seeds of N. caerulescens, Takafumi Mizuno for the seeds of N. japonica, and Rudo Verweij, Rob Broekman, Richard van Logtestijn, Riet Vooijs, and Sandy Goette for technical assistance.
Funding
The studies carried out on Noccaea caerulescens were obtained within the Russian Science Foundation grant (project no. 21-14-00028, https://rscf.ru/project/21-14-00028/). The studies carried out on Noccaea japonica were obtained within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme no. 121040800153-1).
Author information
Authors and Affiliations
Contributions
Authors I.V. Seregin and A.D. Kozhevnikova contributed equally to the work.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Additional information
Abbreviations: Ci—Cira, СМА—Col du Mas de l’Ayre, Co—Le Coulet, Du—Durfort, КН—Krušné Hory, Ku—Kuopio, LA—Les Avinières, La—Lanestosa, LB—Le Bleymard, LC—La Calamine, Le—Lellingen, MP—Monte Prinzera, MS—Moravskoslezské, PB—Puente Basadre, Pl—Plombières, Pr—Prayon, Prem—Prémanon, SB—St-Baudille, SF—St-Félix-de-Palliéres, SLM—St-Laurent-le-Minier (previously Ganges), Vi—Viviez, Wi—Wilwerwiltz (populations of the hyperaccumulator Noccaea caerulescens).
Rights and permissions
About this article
Cite this article
Seregin, I.V., Kozhevnikova, A.D. & Schat, H. Nickel Tolerance and Accumulation Capacities in Different Populations of the Hyperaccumulator Noccaea caerulescens. Russ J Plant Physiol 69, 70 (2022). https://doi.org/10.1134/S1021443722040148
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722040148