Abstract
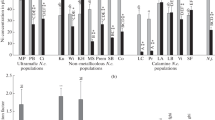
The ability to accumulate nickel (Ni) was compared in hyperaccumulator Noccaea сaerulescens F.K. Mey and excluder Thlaspi arvense L. after a short-term (1, 2, or 3 days) and long-term (8 weeks) exposure. T. arvense and four accessions of N. сaerulescens (La Calamine (LC), Saint Félix de Palliéres (SF), Monte Prinzera (MP), and Lellingen (LE)) were grown on a half-strength Hoagland`s solution in the presence of 25 µM Ni(NO3)2 (N. сaerulescens and T. arvense) and 250 µM Ni(NO3)2 (N. сaerulescens; T. arvense for only 1–3 days). Metal content in the roots and shoots was determined by atomic absorption spectroscopy. The Ni content per unit mass in the roots and shoots of N. сaerulescens in most cases did not differ significantly after the short-term incubation. At 25 µM Ni in the nutrient solution, its content in the roots of LC plants after 2–3 days of incubation was lower than in T. arvense, whereas Ni content in the shoots of these plants was similar. In the plants of other accessions of N. сaerulescens, Ni content in the roots and shoots in most cases was higher than in T. arvense. At 250 µM Ni, the differences in metal content in the roots were insignificant, and its content in the shoots in all the accessions of the hyperaccumulator was much higher than in the excluder. The Ni translocation factor was higher in N. сaerulescens than in T. arvense and exceeded unity only in the plants of MP accession. After the long-term exposure, the Ni translocation factor was higher than 1 in plants of all accessions of N. сaerulescens and decreased in the following order: MP ≈ LC > LE ≥ SF; in T. arvense, it did not exceed 0.3. Upon both long-term and short-term exposure, the ability to accumulate Ni by N. сaerulescens plants of different accessions generally increased in the following order: LC < SF < LE < MP. However, minor changes were observed depending on the duration of exposure and Ni concentration in the medium. Thus, considerable differences in the ability to accumulate Ni among the plants of different accessions of hyperaccumulator N. сaerulescens became apparent as early as during the first days of exposure to Ni and hardly depended on the duration of incubation or metal concentration in the medium. The obtained data confirm the assumption about a constitutive or genetically predetermined ability of plants of different N. сaerulescens accessions to accumulate Ni.



Similar content being viewed by others
REFERENCES
Brooks, R.R., Lee, J., Reeves, R.D., and Jaffré, T., Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants, J. Geochem. Explor., 1977, vol. 7, p. 49.
Reeves, R.D. and Baker, A.J.M., Metal-accumulating plants, in Phytoremediation of Toxic Metals Using Plants to Clean Up the Environment, Raskin, I. and Ensley, B.D., Eds., New York: John Wiley and Sons, 2000, p. 193.
Verbruggen, N., Hermans, C., and Schat, H., Molecular mechanisms of metal hyperaccumulation in plants, New Phytol., 2009, vol. 181, p. 759.
Krämer, U., Metal hyperaccumulation in plants, Annu. Rev. Plant Biol., 2010, vol. 61, p. 517.
Hanikenne, M., Talke, I.N., Haydon, M.J., Lanz, C., Nolte, A., Motte, P., Kroymann, J., Weigel, D., and Krämer, U., Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4,Nature, 2008, vol. 453, p. 391.
Sterckeman, T., Cazes, Y., Gonneau, C., and Sirguey, C., Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction, Plant Soil, 2017, vol. 418, p. 523.
Lin, Y.F. and Aarts, M.G.M., The molecular mechanism of zinc and cadmium stress response in plants, Cell. Mol. Life Sci., 2012, vol. 69, p. 3187.
Ernst, W.H.O., Evolution of metal hyperaccumulation and phytoremediation hype, New Phytol., 2000, vol. 146, p. 357.
Assunção, A.G.L., Schat, H., and Aarts, M.G.M., Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants, New Phytol., 2003, vol. 159, p. 351.
Macnair, M.R., The hyperaccumulation of metals by plants, Adv. Bot. Res., 2003, vol. 40, p. 63.
Deng, T.H.B., Tang, Y.T., Sterckeman, T., Echevarria, G., Morel, J.L., and Qiu, R.L., Effects of the interactions between nickel and other trace metals on their accumulation in the hyperaccumulator Noccaea caerulescens,Environ. Exp. Bot., 2019, vol. 158, p. 73.
Sinclair, S.A. and Krämer, U., The zinc homeostasis network of land plants, Biochem. Biophys. Acta, 2012, vol. 1823, p. 1553.
Assunção, A.G.L., da Costa Martins, P., de Folter, S., Voojis, R., Schat, H., and Aarts, M.G.M., Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens,Plant Cell Environ., 2001, vol. 24, p. 217.
Meharg, A.A., Mechanisms of plant resistance to metal and metalloid ions and potential biotechnological applic-ations, Plant Soil, 2005, vol. 274, p. 163.
Richau, K.H., Kozhevnikova, A.D., Seregin, I.V., Vooijs, R., Koevoets, P.L.M., Smith, J.A.C., Ivanov, V.B., and Schat, H., Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator, Thlaspi caerulescens,New Phytol., 2009, vol. 183, p. 106.
Kozhevnikova, A.D., Seregin, I.V., Erlikh, N.T., Shevyreva, T.A., Andreev, I.M., Verweij, R., and Schat, H., Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens,New Phytol., 2014, vol. 203, p. 508.
Visioli, G., Gullì, M., and Marmiroli, N., Noccaea caerulescens populations adapted to grow in metalliferous and non-metalliferous soils: Ni tolerance, accumulation and expression analysis of genes involved in metal homeostasis, Environ. Exp. Bot., 2014, vol. 105, p. 10.
Seregin, I.V., Erlikh, N.T., and Kozhevnikova, A.D., Nickel and zinc accumulation capacities and tolerance to these metals in the excluder Thlaspi arvense and the hyperaccumulator Noccaea caerulescens,Russ. J. Plant Physiol., 2014, vol. 61, p. 204.
Seregin, I.V., Kozhevnikova, A.D., Zhukovskaya, N.V., and Schat, H., Cadmium tolerance and accumulation in excluder Thlaspi arvense and various accessions of hyperaccumulator Noccaea caerulescens,Russ. J. Plant Physiol., 2015, vol. 62, p. 837.
Callahan, D.L., Baker, A.J., Kolev, S.D., and Wedd, A.G., Metal ion ligands in hyperaccumulating plants, J. Biol. Inor-g. Chem., 2006, vol. 11, p. 2.
Richau, K.H. and Schat, H., Intraspecific variation of nickel and zinc accumulation and tolerance in the hyperaccumulator Thlaspi caerulescens,Plant Soil, 2009, vol. 314, p. 253.
Seregin, I.V. and Kozhevnikova, A.D., Physiological role of nickel and its toxic effects on higher plants, Russ. J. Plant Physiol., 2006, vol. 53, p. 285.
Leitenmaier, B. and Küpper, H., Compartmentation and complexation of metals in hyperaccumulator plants, Front. Plant Sci., 2013, vol. 4, p. 374.
Deng, T.H.B., van der Ent, A., Tang, Y.T., Sterckeman, T., Echevarria, G., Morel, J.L., and Qiu, R.L., Nickel hyperaccumulation mechanisms: a review on the current state of knowledge, Plant Soil, 2018, vol. 423, p. 1.
Lombi, E., Zhao, F.J., Dunham, S.J., and McGrath, S.P., Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense,New Phytol., 2000, vol. 145, p. 11.
Kozhevnikova, A.D., Seregin, I.V., Gosti, F., and Schat, H., Zinc accumulation and distribution over tissues in Noccaea caerulescens in nature and in hydroponics: a comparison, Plant Soil, 2017, vol. 411, p. 5.
Hussain, D., Haydon, M.J., Wang, Y., Wong, E., Sherson, S.M., Young, J., Camakaris, J., Harper, J.F., and Cobbett, C.S., P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis,Plant Cell, 2004, vol. 16, p. 1327.
Bernard, C., Roosens, N., Czernic, P., Lebrun, M., and Verbruggen, N., A novel CPx-ATPase from the cadmium hyperaccumulator Thlaspi caerulescens,FEBS Lett., 2004, vol. 569, p. 140.
Drager, D.B., Desbrosses-Fonrouge, A.G., Krach, C., Chardonnens, A.N., Meyer, R.C., Saumitou-Laprade, P., and Krämer, U., Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels, Plant J., 2004, vol. 39, p. 425.
Craciun, A.R., Meyer, C.-L., Chen, J., Roosens, N., Groodt, R.D., Hilson, P., and Verbruggen, N., Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation, J. Exp. Bot., 2012, vol. 63, p. 4179.
ACKNOWLEDGMENTS
We are grateful to V.B. Ivanov for critical discussion of the obtained results.
Funding
This work was partially supported by the Russian Foundation for Basic Research, project no. 19-04-00369, and the LOCOMET International Research Program.
Author information
Authors and Affiliations
Contributions
A. D. Kozhevnikova and I. V. Seregin made equal contribution to this work.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Additional information
Translated by N. Balakshina
Abbreviations: LC—La Calamine; LE—Lellingen; MP—Monte Prinzera; SF—Saint Félix de Palliéres; PB—Puente Basadre; Pr—Prayon; SLM—Saint Laurent le Minier (formerly Ganges) (accessions of hyperaccumulator Noccaea сaerulescens).
Rights and permissions
About this article
Cite this article
Kozhevnikova, A.D., Seregin, I.V. & Schat, H. Accumulation of Nickel by Excluder Thlaspi arvense and Hyperaccumulator Noccaea caerulescens upon Short-Term and Long-Term Exposure. Russ J Plant Physiol 67, 303–311 (2020). https://doi.org/10.1134/S1021443720020089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720020089