Abstract
Plant developmental processes are very flexible and highly depend on environmental factors. This is largely due to the existence of regulatory mechanisms that systemically control development on the whole plant level. In plants, regulatory peptides produced in the roots have been identified that are activated in response to different factors influencing root system, such as variation in the level of macronutrients (first of all, nitrogen and phosphorus) in the soil, influence of symbiotic microorganisms (soil rhizobial bacteria and arbuscular mycorrhiza fungi), and water deficiency. Among the systemically acting peptides, the most thoroughly investigated are CLE (CLAVATA3/EMBRYO SURROUNDING REGION-related) and CEP (C-TERMINALLY ENCODED PEPTIDES) peptides that are capable of travelling through the xylem from the roots to the shoot and triggering responses via binding to specific receptors operating in the phloem of the leaf. This review focuses on the role of these two groups of peptides in molecular dialog between the root and shoot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Terrestrial plants consist of two main parts: the shoot responsible for carbon assimilation from the air in the course of photosynthesis and the root system engaged in uptake of water and mineral substances from the soil. In order to survive under changing environmental conditions, growth and development of these two parts must be coordinated on the level of whole organism, i.e., systematically. Such coordination implies the existence of specialized signal molecules capable of long-distance transport and mediating communication between different plant organs. Recent research has shown that plant regulatory peptides act as such signal molecules.
By this time, more than 30 classes of peptide phytohormones with various functions have been identified [1, 2]. This review focuses on peptides from families CLE (CLAVATA3/EMBRYO SURROUNDING REGION-related) [3, 4] and CEP (C-TERMINALLY ENCODED PEPTIDES) [5] belonging to the group of posttranslationally modified peptides (PTMP) and often playing antagonistic roles in plant development. All PTMPs are produced as a longer precursor protein out of which an active highly mobile peptide of 12–15 amino acid residues is produced as a result of proteolysis and posttranslational modifications [1]; it can act locally or move to other plant organs as a long distance signal in systemic control of development. Long-distance transport of regulatory peptides occurs along the vascular tissues: a number of peptides produced in the roots can be transported along the xylem from the roots to the shoot where they bind with their receptors located in leaf phloem. A feedback signal from the leaves to the roots is transmitted via phloem with participation of other mobile signals of a different molecular nature. Such a molecular dialog between the root and shoot was predicted more than 30 years ago after investigation of soybean mutants producing excess number of nitrogen-fixing nodules (so-called supernodulating mutants) upon symbiosis with soil rhizobial bacteria [6]. Moreover, in such mutants, nodulation is not suppressed at high concentrations of nitrate in contrast to wild-type plants where nodulation only occurs without an available source of nitrogen in the soil. Grafting experiments have shown that supernodulation phenotype (producing excess number of nodules on the roots) depends in some cases on the shoot genotype: the plants whose shoot was taken from supernodulation mutant plants and engrafted on the root of wild-type plants produced an excess t number of nodules [6]. This result enabled the researchers to assume that the gene that determines symbiotic nodule number operates in the shoot and its product distantly restricts the number of produced nodules, i.e., such regulation depends on long-distance transport of signal molecules and such a regulation is systemic. Long-distance transport of regulatory molecules in plants can be also detected upon investigating the localization of gene transcripts and their protein products: in some cases, lack of transcripts and presence of protein products in the same organ point to the existence of long-distance transport of corresponding proteins. Moreover, long-distance transport of some molecules along the vascular tissues is visualized using fluorescent labels or is monitored by means of radioactive synthetic analogs of regulatory molecules. Finally, it is possible to directly detect regulatory peptides in the xylem sap.
The existence of a systemic control via mobile peptides was also shown for another type of symbiotic interactions, the formation of arbuscular mycorrhiza, as well as for nitrate uptake and response to drought. Whereas nodule formation is a kind of adaptive response to the shortage of soil nitrogen (since the nitrogen fixation by nodule bacteria enriches the plant with nitrogen-containing compounds), the formation of symbiosis with arbuscular mycorrhiza fungi enables plants to uptake more nutrients. especially, phosphorus. Thus, the factors affecting the root system and triggering systemic responses involving long-distance transport of regulatory molecules are related to the main root functions—mineral nutrition and water uptake, which determine physiological status of the whole plant and, in this connection, are regulated on a systemic level. Specific mechanisms underlying a molecular dialog between the root and shoot in regulation of symbiotic reactions, mineral nutrition, and response to drought will be examined below.
CLE PEPTIDES AS SYSTEMIC REGULATORS OF PLANT DEVELOPMENT
CLE are peptide phytohormones that are largely known as regulators of meristem activity. For instance, CLAVATA3 (CLV3), the first described and the most investigated representative of this group, controls stem cell maintenance in the shoot apical meristem (SAM) [4]; CLE40 performs similar functions in the root apical meristem (RAM) [7], while CLE41/44—in cambium [8]. In addition to stem cell regulation in meristems, CLE peptides also participate in differentiation of specific types of cells and tissues [9, 10], in early embryogenesis [11], and in interaction with symbionts [12, 13] and pathogens [14].
CLE genes encode small proteins comprising 100–150 amino-acid residues. CLE proteins have an N-terminal signal domain, central variable part, and a conserved C-terminal CLE domain; some proteins may have several CLE domains [15]. CLE domain comprising 12–13 amino-acid residues corresponds to a mature functional CLE peptide that is cleaved out of precursor protein through proteolysis. Moreover, the activity of CLE peptides requires certain posttranslational modifications: for instance, proline residue at position 7 of the CLE domain is hydroxylated to produce hydroxyproline, to which three residues of arabinose are attached [16]. It was shown that tri-arabinosylation at hydroxyproline improves peptide CLV3’s affinity for its receptor CLV1 [17].
CLE peptides are mobile components of WOX-CLAVATA regulatory systems also comprising receptors of CLE peptides—serine-threonine protein kinases with leucine-rich repeats in the ligand-binding domain (members of family LRR-RLK, Leucine-Rich Repeats containing Receptor-Like Kinases) [18] and a poorly known signal pathway [19] that targets the genes from the WUSСHEL-RELATED HOMEOBOX (WOX) family encoding WOX transcription factors (TF) [20, 21].
CLE peptides are subdivided into several groups based on CLE domain sequences; for instance, Strabala et al. [22] subdivided 32 CLE peptides of Arabidopsis thaliana into groups A–F. According to experimental data, CLE peptides belonging to one group perform identical functions and may substitute for one another mutant backgrounds [22]. A simpler classification is subdivision of CLE peptides into groups A and B depending on their effect on the activity of apical and lateral meristems [8, 23]: peptides from a large group A inhibit the activity of SAM and RAM and do not affect the activity of cambium, whereas peptides from a less numerous group B (also called Tracheary elements Differentiation Inhibitory Factors, TDIF) stimulate cambium activity without affecting the activity of SAM and RAM.
CLE peptides are capable of moving between cells; however, they usually operate locally: in apical meristems and in cambium, they move to neighboring cells (short-distance transport). For instance, in the SAM, the CLV3 gene, is expressed in two upper cell layers of the central zone of the meristem (L1–L2) and migrates to L3 layer where it inhibits expression of the WUSCHEL (WUS) gene responsible for the maintenance of a pool of undifferentiated stem cells [4]. The main receptor of the CLV3 peptide in SAM is CLAVATA-1 (CLV1) receptor protein kinase [24]; CLV3 can also bind to RECEPTOR PROTEINKINASE 1 and 2 (RPK1 and RPK2) operating in SAM [25] and to receptors operating in different types of meristems and differentiated tissues—BARELY ANY MERISTEM 1–3 (BAM1-3) kinases [26] and receptor complex CLAVATA2/CORYNE (CLV2/CRN) [27]. Short-distance transport was also shown for a central regulator of RAM, CLE40, that is produced in columella cells and move to quiescent center of RAM, where it binds to CLV1 and ACR4 (ARABIDOPSIS CRINKLY 4) receptors to suppress the expression of the WOX5 gene [7], as well as for TDIF peptides (CLE41/44 and CLE42) produced in the phloem and perceived by TDIF RECEPTOR/ PHLOEM INTERCALATED WITH XYLEM (TDR/PXY) kinase in cambium to regulated the WOX4 gene [28].
At the same time, it was shown that some CLE peptides are also capable of long-distance transport from the roots to leaves along the xylem mediating plant response to interaction with symbiotic organisms (rhizobia and arbuscular mycorrhizal fungi) and to changes in the content of mineral substances (nitrogen and phosphorus) and drought.
1.1. CLE PEPTIDES IN SYSTEMIC CONTROL OF SYMBIOTIC NODULE DEVELOPMENT AND MYCORRHIZATION
CLE Peptides as Components of the Autoregulation of Nodulation (AON) System
CLE peptides participate in the control of symbiotic nodule development in legumes [12, 13]. The formation and function of nitrogen-fixing nodules are energy-consuming; therefore, these processes are under the systemic control of a plant. In legumes, there exists a system of autoregulation of nodulation (AON) restricting production of excess symbiotic nodules. Certain CLE peptides are mobile components of AON. They are produced in developing nodules and translocated via xylem from the roots to the shoot where they interact with specific receptors—CLAVATA1 (CLV1)-like kinases operating in the leaf phloem: SUPER NUMERIC NODULES (MtSUNN) in M. truncatula, HYPER NODULATION ABERRANT ROOT FORMATION1 (LjHAR1) in Lotus japonicus, PsSYM29 in Pisum sativum) and NODULE AUTOREGULATION RECEPTOR KINASE (GmNARK) in soybean Glycine max [29, 30]. As a result of this interaction, a shoot-derived signal is generated, which inhibits the formation of new nodules on the roots by a negative feedback mechanism. Mutants defective in such CLV1-like kinases produce an abundant number of nodules (supernodulation) [29, 31–33]; the grafting experiments have shown that supernodulating phenotype depends on the shoot genotype: if the shoot of the mutant plant is engrafted upon the root of a wild-type plant, the roots of such plants will show supernodulation after inoculation with rhizobia.
CLE peptides acting as inductors of AON were described in different model legumes: MtCLE13 and MtCLE12 in M. truncatula [13]; LjCLE-RS1, LjCLE-RS2, and LjCLE-RS3 (from CLE-ROOT SIGNAL) in L. japonicus [12]; GmRIC1 and GmRIC2 (from RHIZOBIUM INDUCED CLE) in G. max [30, 34]. CLE genes activated upon nodulation were also identified in P. sativum [35]; however, their role as participants in AON has not been investigated so far.
As was shown in M. truncatula, expression of the MtCLE13 and MtCLE12 genes inducing AON is activated rather early in response to rhizobial infection: 6 and 48 h after inoculation, respectively [36]. The promoter activity of MtCLE13 visualized using pM-tCLE13:GUS is observed at early stages of primordium development in proliferating cells of cortex and pericycle, while its expression in the mature nodule is concentrated in the apical part of the nodule: in nodule meristem, in the zone of early infection, and at the tips of vascular bundles. Similar expression pattern was observed for the MtCLE12 gene; however, the activity of pMtCLE12:GUS suggests that its expression is triggered later, in the cells of already formed nodular primordium [13].
Function of CLE peptides involved in nodulation (in particular, their binding to receptors) depends on posttranslational modifications, hydroxylation, and tri-arabinosylation of conserved proline residues in the CLE domain: in M. truncatula and pea, mutants defective in hydroxyproline-O-arabinosyl transferase (rdn1, root determined nodulation1 in M. truncatula and nod3, nodulation 3, in P. sativum) exhibit a supernodulation phenotype that (according to grafting experiments) depends on the root part of the plant [37, 38]. In M. truncatula, hydroxyproline-O-arabinosyl transferase RDN1 is required for posttranslational modification of MtCLE12 but not MtCLE13 [39].
The long-distance transport of CLE peptides involved in AON was suggested by the effect of overexpression of CLE genes in CLV1-like kinase mutant background [12, 13]. For instance, in wild-type plants, overexpression of symbiosis-specific CLE genes (in particular, MtCLE12 and MtCLE13 in M. truncatula LjCLE-RS1 and LjCLE-RS2 in L. japonicus) in transgenic roots obtained by Agr-obacterium rhizogenes-mediated transformation inhibited nodulation not only in transgenic roots with CLE overexpression but also in non-transgenic roots. On the contrary, in Mtsunn and Ljhar1 mutants defective in CLV1-like kinase, overexpression of symbiosis-specific CLE genes (MtCLE12/MtCLE13 and LjCLE-RS1/LjCLE-RS2, respectively) did not suppress nodulation [12, 13]. These results suggest that shoot-acting CLV1-like kinases are required for the perception of root-derived CLE peptides. For LjCLE-RS2, xylem transport and binding to CLV1-like kinase was shown [40]. LjCLE-RS2 was identified in xylem sap collected from the shoot, and chemically synthesized LjCLE-RS2 peptide with tri-arabinosylated hydroxyproline could suppress nodulation upon its application directly to cotyledon leaves. Moreover, radioactive synthetic LjCLE-RS2 specifically bound to LjHAR1 protein produced in tobacco cells [40]. Thus, LjCLE-RS2 peptide synthesized in the root travels via xylem to the shoot where it binds to its receptor—CLV1-like kinase LjHAR1. It is assumed that CLE peptides involved in AON function in a similar way in other legumes binding to CLV1-like receptor kinase in the shoot and trigger a signaling cascade to suppress nodulation.
Along with CLV1-like kinase, the receptor complex that binds root-derived CLE peptides is believed to include other proteins. Using a BiFC (bimolecular fluorescence complementation) approach, it was shown that MtCRN and MtCLV2 proteins interact with CLV1-like kinase MtSUNN [41], and mutation in the MtCLV2 gene also results in a supernodulation phenotype [33]. However, in L. japonicus LjCLV2 and LjHAR1 did not show a direct interaction due to a different intracellular localization: the LjHAR1 protein is localized on plasma membrane and LjCLV2—on endoplasmic reticulum [33]. In L. japonicus, it was shown that the LjHAR1 protein interacts with the KLAVIER (KLV) receptor-like kinase and mutation in the LjKLV gene also results in shoot-controlled supernodulation [42]. Collectively, these data indicate that CRN, CLV2, and KLV kinases may participate in the reception of CLE involved in AON.
Activation of CLV1-like kinase by root-derived CLE peptides triggers a feedback shoot-derived signal inhibiting nodulation (SDI, shoot-derived inhibitor) [30]. Such shoot-derived inhibitor seems to have a complex nature, involving the changes in phytohormone biosynthesis and transport from the shoot to the root. For instance, CLV1-like kinase activation reduced auxin transport from the shoot to the root [43]; at the same time, it is known that jasmonic acid and abscisic acid (ABA) also mediate the inhibition of nodulation in response to AON [44, 45]. In addition, AON triggers the activation of cytokinin biosynthesis in the shoot, and shoot-derived cytokinin suppresses nodulation on the roots [46].
Among the factors acting downstream of CLV1-like kinase, there is a TOO MUCH LOVE (TML) protein containing kelch-repeats (motifs responsible for protein-protein interactions) and an F-box (motif also responsible for protein-protein interactions and found in the components of ubiquitin-ligase complexes mediating ubiquitin-dependent protein degradation) [47]. Mutation in the TML gene results in the emergence of a supernodulation phenotype that depends on the root part of plant, which indicates that the TML protein acts in the root [48]. The TML protein is localized in the nucleus and is supposed to be involved in the suppression of nodulation via ubiquitin-dependent degradation of TFs regulating nodule development [48]. In L. japonicus, the transcripts of the LjTML gene are direct targets for microRNA miR2111 in vivo [49]. The level of miR2111 decreases in response to rhizobial infection both in the roots and shoots of inoculated plants [49]. Overexpression of the MIR2111-3 gene (a precursor of miR2111) resulted in hyperinfection and increased number of nodules, which were found to be smaller and less developed than wild-type ones. According to its promoter activity, the MIR2111-3 gene is predominantly expressed in the leaf phloem, whereas its direct target—TML transcripts—were found only in rhizobia-inoculated roots but not in the leaves. A number of experiments with mechanical separation of the shoot and root, as well as detection of mature miR2111 in the phloem exudate, enabled Tsikou et al. [49] to suggest a systemic effect of miR2111 in regulation of nodulation: being transported from the shoot, miR2111 causes degradation of TML transcripts in the absence of rhizobia, whereas rhizobial infection makes it possible to accumulate TML transcripts owing to a reduction in miR2111 level; as a result, nodulation is suppressed. Moreover, in Ljhar1 mutant upon rhizobial infection, the level of miR2111 was higher both in the leaves and roots as compared with wild-type plants. These data suggest that CLV1-like kinase encoded by gene LjHAR1 mediates decrease in miR2111 level upon rhizobial infection, which results in an increase of TML transcripts and consequently inhibits nodulation [49]. Similar results on AON-dependent decrease of miR2111 level upon rhizobial infection were also obtained for M. truncatula: in Mtsunn mutant bearing a mutation in the gene orthologous to LjHAR1, the level of miR2111 did not decrease in the shoots or roots upon rhizobial infection and inhibition of miR2111 by means of artificial antagonistic microRNA was sufficient to complement supernodulation genotype in Mtsunn mutant (i.e., caused a reduction in nodule number to the level of wild-type plants) [50].
Recently, it was found that regulation of miR2111 level in M. truncatula also involves COMPACT ROOT ARCHITECTURE 2 (CRA2) receptor kinase—a receptor of CEP peptides in the shoot [50]. Thus, microRNA miR2111 is a systemic regulator of nodulation integrating the signal from receptors of two groups of peptide hormones, CLE and СЕР (see below), that have an antagonistic effect on symbiotic nodule development in legumes.
It was shown that activation of CLE gene expression upon nodulation depends on NODULE INCEPTION (NIN) TF—a key regulator of symbiotic nodules development [51]. Using chromatin immunoprecipitation (ChIP-seq), it was shown that LjNIN TF directly binds to the promoters of LjCLE-RS1 and LjCLE-RS2 genes and activates their expression [51]. At the same time, expression of the NIN gene itself and NIN targets—CLE genes, suppressing nodulation—is induced by cytokinin [51, 52]; this suggests that it is NIN which mediates CLE activation in response to cytokinin [51]. Overexpression of LjNIN in transgenic roots obtained by A. rhizogenes-mediated transformation resulted in a systemic suppression of nodulation both in transgenic roots overexpressing NIN and in non-transgenic roots, with such a suppression of nodulation being associated with an increase of the LjCLE-RS1 and LjCLE-RS2 genes. Systemic inhibition of nodulation upon NIN overexpression was not observed in Ljhar1 and Ljtml-1 mutants, which suggests that systemic suppression of nodulation involving NIN is mediated by AON. Similarly, NIN overexpression systemically inhibited its own expression, the NIN gene itself, which was shown by the activity promoter:GUS fusion; in Ljhar1 mutants, such suppression was not observed. These data indicate that AON negatively regulates NIN expression on a transcriptional level, which results in the expression inhibition of NIN target gene responsible for activation of cortical cell divisions upon primordia development and, as a result, suppresses nodulation [51].
Thus, NIN TF triggers AON and, at the same time, NIN itself is a target for systemic regulation of nodulation; CLE peptides activated by NIN bind to CLV1-like receptor kinases in the shoot and promote a feedback signal that inhibits nodulation, particularly, via the inhibition of NIN expression. Therefore, NIN-CLE-СLV1-like kinase regulatory module resembles the WUS-CLV3-CLV1 regulatory system operating in the shoot apical meristem, wherein WUS TF directly triggers the expression of the CLV3 gene whose product inhibits WUS expression via receptor complexes involving CLV1 [4].
Role of CLE Peptides in Nitrate-Mediated Inhibition of Nodulation
Nodulation costs the plant much energy resources. If the soil contains available nitrogen compounds, the plant will predominantly use them to maintain its mineral status. In this relation, plants evolved a regulatory mechanism enabling them to control the emergence of nodules depending on availability of nitrogen-containing components in the soil and nitrate in particular.
A number of CLE peptides activated upon symbiosis and triggering AON are also induced by nitrate treatment, thereby mediating nitrate-dependent suppression of symbiotic nodule development [12, 53]. For instance, in L. japonicus the expression of two out of three CLE genes induced by rhizobia, is also induced in response to nitrate treatment (LjCLE-RS2 and LjCLE-RS3) [12].
In soybean Glycine max, two groups of CLE peptides were described differing in the mechanism of activation of their expression. In addition to RIC peptides (GmRIC1 and GmRIC2) induced by rhizobia and involved in systemic control of nodulation via shoot-acting CLV1-like kinase GmNARK, nitrate-induced CLEs belonging to a NIC group (NITRATE INDUCED CLE) have been also described [54]. GmNIC1 and GmNIC2 expression is activated in response to nitrate present in the soil and results in the inhibition of nodule formation. Reciprocal graftings showed that nodule number in plants with a wild-type rootstock was significantly decreased upon nitrate treatment as compared with plants with a rootstock originated from the Gmnark genotype [54]. This result indicates that nitrate inhibition of nodulation depends on root-acting GmNARK receptor kinase. Accordingly, in contrast to systemically operating RIC peptides, GmNIC1 and GmNIC2 peptides mediate local response to nitrate treatment, since overexpression of the GmNIC1 and GmNIC2 genes in transgenic roots did not suppress nodulation on non-transgenic roots in composite plants with GmNIC1 and GmNIC2 overexpressing transgenic roots obtained by A. rhizogenes-mediated transformation. At the same time, overexpression of GmNIC1 and GmNIC2 suppressed nodulation on wild-type roots but not in the Gmnark mutants [54, 55]. These results indicate that GmNIC1 and GmNIC2 peptides suppress nodulation locally via root-acting GmNARK [54, 55]. Thus, in contrast to L. japonicus, the nitrate-dependent mechanism suppressing nodule development in soybean operates locally via root-acting CLV1-like kinase. Specific mechanisms ensuring root reception of CLE by GmNARK remain to be elucidated; the issue as to why, in contrast to nitrate-activated GmNIC peptides, GmRIC peptides activated by rhizobia do not act via root receptor but operate systemically via GmNARK that functions in the shoot is to be addressed.
In L. japonicus, the expression of nitrate-inducedCLE, LjCLE-RS2 in particular, is activated by LjNRSYM1 (NITRATE UNRESPONSIVE SYMBIOSIS 1) TF belonging to the family of NIN-like proteins (NLP). NLP TFs were also described in other plants, in particular in A. thaliana, as regulators of nitrate response [56]; they comprise a conserved DNA-binding RWP-RK domain involved in their interaction with nitrate response elements (NRE) within regulatory sequences of nitrate-induced genes, and PB1 domains responsible for protein-protein interactions [57]. Ljnrsym1 mutant can produce nodules at high nitrate concentrations [58], which points to its role in nitrate-dependent inhibition of nodulation.
However, the level of LjNRSYM1 expression did not change after nitrate treatment and inoculation with rhizobia. The activity of NRSYM1 TF is assumed to be regulated owing to its posttranslational modifications induced by nitrate [58]. It was found that nitrate influences on N-terminal domain of NLP proteins: in Arabidopsis, nitrate treatment affected NLP intracellular localization and stimulated its accumulation in the nucleus depending on phosphorylation of an amino-acid residue in the N-terminal domain [59] (see 1.2). Similar nitrate-mediated translocation from the cytoplasm to the nucleus upon nitrate treatment was described for the LjNRSYM1 protein [58]. The ability to change conformation and migrate to the nucleus under exposure to nitrate distinguishes NLPs from NIN protein regulating development of symbiotic nodules, which lost the ability to respond to nitrate in the course of evolution [60]. The LjNLP1 protein was shown to bind to NRE in the promoters of target genes whose structure is identical to the sequences bound by the LjNIN protein (NIN-binding sites, NBS) [60]. LjNRSYM1 also directly binds to NBS/NRE in the promoter of the LjCLE-RS2 gene [58].
The role of NLP TF in regulating of nitrate-induced gene expression was also investigated in M. truncatula [61]. It was shown that MtNLP1 that is responsible for the activation of nitrate-regulated genes (in particular, of the NIR1 gene encoding nitrite reductase and the NRT2.1 gene encoding nitrate transporter) can bind to MtNIN TF. The authors proposed a model where NLP TF in an inactive state is predominantly located in the cytoplasm and, in response to the increase of nitrate concentration, moves to the nucleus where it forms heterodimer with NIN, blocking its function by preventing the activation of target gene expression, thereby inhibiting nodule formation [61].
CLE Peptides as Systemic Regulators of Mycorrhiza Formation
Many terrestrial plants can form a symbiosis with arbuscular mycorrhizal fungi, and the plants can receive more mineral substances (and especially phosphorus) from the soil owing to mycorrhiza. Recently, CLE peptides activated upon mycorrhization and phosphate treatment have been identified in legumes; they systemically suppress further mycorrhiza development via the same receptor that is involved in AON [62]. For instance, expression analysis of genes activated upon M. truncatula mycorrhization by Rhizophagus irregularis showed higher expression of the MtCLE16, MtCLE45, and MtCLE53 genes. Moreover, it was shown that elevated content of phosphate in the medium (2 mM) activated the expression of MtCLE32 and MtCLE33. Overexpression of the MtCLE33 and MtCLE53 genes resulted in a suppression of mycorrhization due to the reduction of strigolactones—the activators of arbuscular mycorrhiza development, suggesting that genes involved in strigolactone biosynthesis are the targets of CLE-mediated signaling [62].
In contrast to wild-type plants, overexpression of the MtCLE33 and MtCLE53 genes in Mtsunn mutant did not reduce mycorrhization or suppressed biosynthesis of strigolactones [62]. Therefore, MtSUNN receptor kinase controls not only legume-rhizobial symbiosis but also regulates another type of symbiosis - arbuscular mycorrhiza. In the cases of legume-rhizobial symbiosis and arbuscular mycorrhiza, activation of MtSUNN receptor depends on different CLE peptides (MtCLE12/MtCLE13 and MtCLE33/MtCLE53, respectively), and the mechanisms of symbiotic suppression triggered by MtSUNN also differ for different symbiotic interactions. Moreover, it was shown that a mutation in the MtSUNN gene also alters nitrate uptake and its translocation to the shoot, which was shown by transcriptome analysis of Mtsunn аnd by a reduced nitrate content in the shoot of Mtsunn mutant as compared with wild-type plants in 24 h after the addition of nitrate to the medium [63]. Particular factors that determine the specificity of responses activated by a central regulator (MtSUNN kinase) are still unknown in each case.
1.2. NITRATE-DEPENDENT REGULATION OF LATERAL ROOT FORMATION BY CLE PEPTIDES AND OTHER NITRATE-REGULATED PROCESSES OF PLANT DEVELOPMENT
CLE peptides mediate nitrate-dependent responses in plants not only associated with symbiosis but also in other developmental process. It is known that nitrate affects the formation of the root system [64, 65] and regulates activity of the shoot meristem and flowering time [66]. For instance, in legumes, mutations in CLV1-like kinase gene involved in AON not only cause supernodulation but also impair root architecture: in LjHar1 mutants the main root is shorter and lateral root number is increased [67]. In Arabidopsis, nitrate response presumably depends on CLV1 receptor kinase—a central regulator of SAM development; in clv1 mutants, in contrast to wild-type plants, nitrate deficiency did not suppress lateral root development [64].
In Arabidopsis, nitrate-regulated CLE peptides involved in control over development of lateral roots were also described [64]. For instance, Araya et al. [64] showed that the AtCLE1/3/4/7 genes of Arabidopsis are activated upon nitrate deficit and are mainly expressed in pericycle cells; their overexpression suppresses lateral root development. AtCLE3 overexpression caused a suppression of lateral root growth in wild-type plants but its inhibitory effect on lateral root growth was not shown in Atclv1 mutant. This suggests that AtCLE1/3/4/7 peptides suppress lateral root growth via AtCLV1 receptor kinase under nitrogen deficiency. The authors assumed that, since AtCLV1 protein is localized in the root phloem, the lateral roots growth is regulated locally by AtCLEs via root-acting AtCLV1 receptor kinase; putative long-distance transport of AtCLE peptides was not investigated [64].
At the same time, NIN-like proteins AtNLP6 and AtNLP7 were identified in Arabidopsis which are responsible for the activation of nitrate-regulated genes. For instance, it was shown that, in the presence of nitrate in the medium, AtNLP7 is translocated to the nucleus where it binds to nitrate-regulated elements (NRE) in the sequences of target genes [57]. Nuclear retention of NLP7 is depends on its phosphorylation at Ser205 residue by Ca2+-sensor protein kinases (CPKs) CPK10, CPK30, and CPK32 [68]. Nitrate-dependent activation via AtNLP6/7 was described for a number of genes controlling different aspects of plant development: in particular, for the CYCLINB1;1 gene encoding сyclin B [69] and for a number of flowering regulating genes [66].
Apart from CLE peptides, cytokinins may also act as long-distance transport molecules produced in response to nitrate. It is known that, in Arabidopsis root, the expression level of IPT genes controlling the first stage of cytokinin biosynthesis from adenosine phosphates directly depends on nitrate concentration in the medium [70]. It was shown that long-distance root-derived cytokinin transport to the shoot regulates nitrate-dependent broadening of WUS expression zone, which leads to an increase of SAM size [71].
1.3. CLE PEPTIDES AS REGULATORS OF DROUGHT RESPONSE
Under a a water deficiency in soil, plants experience stress and respond to it by stomatal closure, which reduces water loss and helps plants to survive the period of drought. This response is mediated by ABA produced in the leaves under drought. ABA biosynthesis in the leaves was found to be regulated by CLE peptides produced in the roots. In Arabidopsis root treatment with CLE25 synthetic peptide stimulated the expression of the AtNCED3 gene (NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3) encoding a key enzyme of ABA biosynthesis under drought and, as a result, caused stomatal closure in the leaves. AtCLE25 root-to-shoot transport was confirmed using radioactively labeled synthetic AtCLE25 whose accumulation was observed in the leaves after root treatment. AtCLE25 promoter activity was observed in lateral roots, root tips, vascular tissues of leaves, and in root procambium. In mutants defective in the AtCLE25 gene obtained by CRISPR-Cas9-mediated editing and in plants with AtCLE25 RNA interference exposed to drought, the expression of the AtNCED3 gene was not activated; therefore, ABA accumulation in the leaves of such plants was not observed upon dehydration, in contrast to wild-type plants. Moreover, mutants defective in AtCLE25 showed a greater loss of water and were more sensitive to drought than wild-type plants. In order to reveal possible receptors of AtCLE25 peptide, AtCLE25 effect was accessed in a number of mutants defective in genes encoding CLE receptors. It was found that in the leaves of bam1-5 bam3-3 double mutant (which also demonstrates increased drought sensitivity) ABA biosynthesis was not activated upon AtCLE25 treatment. Moreover, grafting experiments have shown that it was the shoot that determined plant sensitivity to AtCLE25. These data suggest that BAM1 and BAM2 receptor kinases may act as AtCLE25 receptors in the leaves [72].
Recently it was found that, along with its participation in drought response, AtCLE25 peptide is necessary for phloem development: mutation in the AtCLE25 gene resulted in a delay of phloem cell differentiation, and substitutions of conserved glycine residues by threonine at the 6-th position (G6T) in the CLE domain of AtCLE25 also disturbed phloem development and resulted in a shortened root [73]. Analysis of AtCLE25-insensitive mutants revealed that СLERK (CLE-RESISTANT RECEPTOR KINASE) receptor kinase is a putative receptor for AtCLE25 in the root, that forms a complex with CLV2 [73]. Thus, along with its role in long-distance transport and systemic drought responses via BAM1/BAM3 receptors in the leaves, AtCLE25 peptide also act locally in the root via СLERK/CLV2 receptor complex regulating phloem formation. Long-distance transport of AtCLE25 via xylem to the shoot in response to drought described by Takahashi et al. [72] apparently requires its movement from the phloem to xylem cells.
2. CEP PEPTIDES AS SYSTEMIC REGULATORS OF PLANT DEVELOPMENT
In Arabidopsis, CEP peptides were identified in silico in screening of protein families containing a structure characteristic of PTMP—a short C-terminal conserved domain. The processing of СЕР peptides is associated with proteolysis and posttranslational modifications in the conserved domain (hydroxylation of proline residues) [5]. Mature CEP peptides consist of 15 amino acid residues and contain 2–4 residues of hydroxyproline. In contrast to CLE peptides that were found even in green algae [74], СЕР peptides were identified only in seed plants: for instance, 15 CEP genes expressing predominantly in the stele of lateral roots were detected in Arabidopsis [75].
Functions of СЕР peptides are apparently related to root system development, including control of lateral root and nodule development and response to abiotic stress, especially to the deficit of nitrogen; at the same time, they can act systemically at long distances from the place of their synthesis (see below).
Regulation of CEP Expression by Nitrate and Other Agents
In Arabidopsis, genes of the CEP family are predominantly expressed in the root and stem being activated upon a nitrogen deficiency in the medium and after exposure to other stress factors, such as increased amount of sodium chloride in the medium and excess carbon dioxide. AtCEP3 was shown to be activated in the root upon nitrogen deficiency. Moreover, a mutant defective in the AtCEP3 gene exhibited more developed root system under different stress factors (for example, nitrogen deficiency or salination) as compared with wild-type plants; on a standard medium, it did not show differences comparing with wild-type plants [76]. These data suggest that CEP peptides (and especially AtСЕР3) act as negative regulators of root-system development under stress conditions.
2.1. CEP RECEPTORS—SYSTEMIC REGULATORS OF RESPONSE TO NITROGEN DEFICIENCY
The receptors for CEP peptides were identified by Tabata et al. [77]: radioactive synthetic peptide AtCEP1 in vitro specifically bound to AtCEPR1 and AtCEPR2 (CEP RECEPTOR) kinases from LRR-RLK family the, wherein mutants defective in the AtCEPR1/2 genes was insensitive to CEP1 treatment in root growth inhibition assay. AtCEPR1 and AtCEPR2 promoter activities were detected in vascular tissues of the leaves, main root, and lateral roots. cerp1 cerp2 double mutants demonstrated longer lateral roots. A similar but less pronounced phenotype was also observed in cerp1 single mutant but not in cerp2 mutant, which suggesting CEPR1 plays a leading role in AtCEP1 reception and triggering subsequent reactions resulting in suppression of root growth. In addition, cerp1 cerp2 mutants showed a number of characteristics typical for plants exposed to nitrogen starvation: accumulation of anthocyans, pale green color of leaves, and shoot shortening; therefore, the authors assumed that AtCEP1 and its receptors may regulate the expression of genes involved in nitrogen uptake and accumulation in plants. Indeed, in cerp1 cerp2 mutants, expression of the NRT2.1 gene encoding nitrate transporter was considerably reduced and expression of NRT3.1 and NRT1.1 genes was slightly lower in comparison with wild-type plants. Treatment with exogenous AtCEP1 activated the expression of the NRT2.1 gene even at a high content of nitrogen in wild-type plants but failed to do it in mutants defective in the AtCEPR1 gene. Thus, AtCEP1 peptide mediates response to nitrogen deficiency in the medium via AtCERP1 receptor [77].
Split-root technique that envisages subdivision of the root system into two parts and their exposure to different conditions (for instance, one half of the root system is accommodated in the medium with a high content of nitrogen and the other is exposed to a low level of nitrogen) made it possible to show that nitrogen starvation experienced by one half of the root system may stimulate lateral root development and increase the expression of the genes encoding nitrate transporters in the second half of roots exposed to the medium with a high nitrogen content in order to compensate for overall nitrogen deficiency [78]. Such a systemic response turned out to be mediated by CEP peptides and their receptors [77]. For instance, in contrast to wild-type plants, cepr1 cepr2 mutants did not show a systemic activation of the NRT2.1, NRT3.1, and NRT1.1 genes encoding nitrate transporters in the roots placed in the medium with a high nitrogen (10 mM \({\text{NO}}_{3}^{ - }\), split root technique). Moreover, in the high nitrogen medium, when one part of the root system was treated with a synthetic AtСEP1 peptide the activation of NRT2.1 expression was observed in treated and untreated roots, which indicates that AtCEP1 mediates systemic activation of the genes encoding nitrate transporters.
Since the AtCEPR1 and AtCEPR2 genes are expressed both in the root and in the shoot, it was interesting to find out whether AtCEP1 peptide affects nitrate transport locally or it is transported to the shoot. AtCEP1 was shown to act systemically via shoot-acting receptors, since in grafting experiments when the shoot of cepr1-1 cepr2-1 double mutant was grafted onto the rootstock of wild-type plants, treatment one part of the root system in split root system by the AtCEP1 peptide did not activate the expression of NRT2.1 in any part of the root system [77].
What factors mediate signal transduction from shoot-acting CEP receptors to the root? Ohkubo et al. have shown that, when Arabidopsis roots were treated with AtCEP1 peptide, AtCEPR1-dependent activation of the CEP DOWNSTREAM 1 (CEPD1) and CEPD2 genes encoding polypeptides from family 10 of class III glutaredoxins occurs in the cells of leaf phloem [79]. Analysis of plant overexpressing the AtCEPD1 and AtCEPD2 genes, as well as cepd1 cepd2 double mutant plants, has shown that AtCEPD1 and AtCEPD2 are responsible for systemic activation of NRT2.1 expression in the roots but not two other genes (NRT3.1 and NRT1.1) whose expression was earlier shown to be activated by AtCEP1 treatment [77, 79]. Promoter activities of the AtCEPD1 and AtCEPD2 genes was observed in the leaf phloem. AtCEPD1 and AtCEPD2 transcripts were not detectable in the roots even after CEP1 treatment. At the same time, a hybrid protein GFP-AtCEPD1 was localized in the root vasculature, in phloem cells, and in the cells of endodermis, and its content increased both after AtCEP1treatment and upon nitrogen deficiency [77]. These data indicate that AtCEPD polypeptides act as mobile signal molecules transported through the characteristic phloem from the shoot to root in response to AtCEPR1 receptor activation in the shoot. This conclusion was additionally confirmed by grafting experiments where GFP-AtCEPD1 hybrid protein was detected in the root system of cepd1-1 cepd2-1 mutant when GFP-AtCEPD1 hybrid gene was expressed in the shoot part of a plant [79].
Moreover, Ota et al. [80] recently detected two more genes with a high similarity to AtCEPD1 called AtCEPD-like 1 (CEPDL1) and CEPDL2. Their overexpression also caused activation of NRT2.1 expression in the roots even in nitrogen-reach medium (10 mM \({\text{NH}}_{4}^{ + }\) and 10 mM \({\text{NO}}_{3}^{ - }\)). Transcriptome study of plants overexpressing AtCEPDL2 found that AtCEPDL2 stimulates expression of the genes encoding transporters with high-affinity nitrate uptake, such as NRT2.1, NRT2.2, NRT2.4, and NRT3.1 as well as NRT1.5 that loads nitrate into xylem for its translocation from the root to shoot [80]. As its homologs AtCEPD1 and AtCEPD2, AtCEPDL2 protein is transported from the shoot to the root through the phloem, which was revealed by the analysis of promoter activity and fluorescence of GFP-AtCEPDL2 hybrid protein. However, in contrast to AtCEPD1 and AtCEPD2, AtCEPDL2 expression in the shoot was activated upon nitrogen deficiency irrespective of AtCEPR1 receptor activity, and was detected even in the shoots detached from the roots at low nitrogen concentrations. This suggests that AtCEPDL2 expression is regulated by nitrogen content in the shoot [80].
Thus, regulation of nitrate uptake system in the plant root is rather complicated; it is induced in a parallel by two pathways: upon nitrogen limitation in the shoot via AtCEPDL2 and under nitrogen deficiency in the soil via AtCEP mobile peptides produced in the roots and their shoot-acting AtCEPR receptors [80].
CEP Peptides as Local Regulators of Lateral Root Development
Along with systemic responses to the nitrogen deficiency, CEP peptides are assumed to participate in local regulation of lateral roots development. For instance, the expression of the AtCEP5 gene in Arabidopsis is suppressed by auxin and associated with early stages of lateral root development: AtCEP5 is expressed in pericycle cells near the phloem pole, i.e., close to the sites of lateral root initiation but not within them (in Arabidopsis, the lateral root is initiated in pericycle cells opposite to the xylem pole). According to Roberts et al. [81], the AtCEPR1 gene expressing in the same cells encodes a putative receptor of AtCEP5 peptide since cepr1 mutants were insensitive to exogenous treatment with AtCEP5 synthetic peptide. The data on AtCEP5 overexpression and RNA interference, together with the results on exogenous AtCEP5 treatment have shown that AtCEP5 suppresses lateral root development. In a mutant defective in the AtCEPR1 gene (also known as XIP1, from XYLEM INTERMIXED WITH PHLOEM 1), the number of lateral roots was reduced, suggesting that AtCEP5 peptide locally inhibits lateral root development by a negative regulation of AtCEPR1/XIP1 action—a positive regulator of lateral root development [81]. The role of CEP peptides in local regulation of lateral root development was also shown for M. truncatula: overexpression of the MtCEP1 gene in hairy root culture (lacking shoots) suppressed lateral root development [82].
2.2. CEP PEPTIDES IN NODULATION
Nitrogen-fixing nodules emerge on the roots of legumes as a result of symbiosis with rhizobial bacteria under low nitrogen in the soil, and in this respect, nodulation itself can be considered as a response to nitrogen deficiency. In M. truncatula, the treatment with MtCEP1 peptide, as well as overexpression of MtCEP1, stimulated the formation of nodules and simultaneously suppressed lateral root initiation [83]. Moreover, treatment with exogenous MtCEP1 peptide and MtCEP1 overexpression caused the formation of so-called ССР (circumferential cell proliferations) sites—periodic swellings arising on the root as a result of proliferation of pericycle, cortical, and epidermal cells. In CCP-sites, lateral roots with developmental arrest were observed; other CCP sites were developed into symbiotic nodules upon rhizobial inoculation [83]. Nodules formed upon MtCEP1 overexpression and MtCEP1 treatment developed faster, they were larger, and showed a higher nitrogenase activity than control ones even at a high concentration of nitrate in the medium (up to 25 mM) [83].
In M. truncatula, cra2 (compact root system architecture 2) mutant with a shortened root and numerous lateral roots was described [84]. In addition, cra2 mutant had a reduced number of symbiotic nodules, and the suppression of nodulation was observed at early stages of nodule primordium development (on the first to third day after inoculation), and this effect was irrespective of cra2 effect on root shortening. The MtCRA2 gene is expressed in shoot and root vasculature, in of lateral root and nodule primordia, and in mature nodules: in nodule apical meristem and in peripheral vascular bundles. Grafting experiments have shown that MtCRA2 locally (in the root) regulates the development of lateral roots and systemically (with participation of the shoot) controls the development of symbiotic nodules [84]. The MtCRA2 gene encodes a receptor-like kinase with leucine-rich repeats (LRR-RLK) and has high sequence similarity with the AtXIP1 gene in Arabidopsis [84] responsible for CEP peptides reception [77]. Mutant defective in the MtCRA2 gene turned out to be insensitive to MtCEP1. Mohd-Radzman et al. [82] showed that MtCEP1 effect on symbiotic nodule development and lateral root development depends on MtCRA2, which, together with its high sequence similarity to AtXIP1, points to a possible role of MtCRA2 in MtCEP reception.
MtCEP1 peptide treatment of a set of mutants with altered nodule development (defective in the genes encoding the components of rhizobia-activated signaling cascade) failed to induce nodulation but suppressed lateral root development, which points to the existence of independent pathways for regulation of nodule development and lateral root development by MtCEP1. For instance, in M. truncatula supernodulating Mtsunn mutant defective in AON, MtCEP1 treatment resulted in even greater increase in nodule number and caused a reduction in the number of lateral roots as compared with control plants; moreover, the root zone where nodules were developed became wider [82].
On the contrary, in another supernodulation mutant Mtein2/sickle (skl) which is defective in ethylene signal transduction, demostrates hyperinfection, and forms excessed number of immature and often fused nodules, MtCEP1 treatment or the MtCEP1 gene overexpression did not cause an increase in number of nodules or broadening the nodulation zone within the root. MtEIN2 encodes an ortholog of the EIN2 protein in Arabidopsis—a central regulator of cell response to ethylene [85]. In Medicago truncatula, it also inhibits nodulation and mediates a negative influence of ethylene on initiation and development of symbiotic nodules. Interestingly, that MtCEP1 treatment of wild-type plants also resulted in rhizobial hyperinfection and increased the formation of fused nodules, with a larger percentage of nodules being initiated opposite to the phloem pole (in contrast to control plants where nodules are initiated opposite to the xylem pole). All these features are also characteristic of the skl mutant [86]. These data and the results on combined treatment with MtCEP1 peptide and ethylene precursor ACC suggest that the stimulatory MtCEP1 effect on nodulation may be mediated through the suppression of MtEIN2-mediated ethylene signaling [82]. Collectively, the data obtained by Mohd-Radzman et al. [82] indicate that the MtCEP1 peptide negates the inhibitory effect of ethylene on nodule initiation via MtCRA2-dependent mechanism. Thus, MtCEP1 and its putative receptor MtCRA2 regulate lateral root and nodule initiation by activating two different regulatory pathways: the first pathway is associated with a systemic (via shoot-acting MtCRA2) activation of nodulation via the suppression of ethylene-MtEIN2-mediated inhibition of nodulation; the second pathway is related to a local (via root-acting MtCRA2) inhibition of lateral root initiation independent of ethylene signaling.
Quite recently, one more mechanism was described by which MtCEP1 and its putative receptor MtCRA2 can stimulate nodulation [50]. Gautrat et al. [50] identified the MtCEPD1 and MtCEPD2 genes M. truncatula, homologs of AtCEPD geness, whose products in Ar-abidopsis are mobile polypeptides that mediate a shoot-to-root transduction of the signal from CEP receptors. MtCEPD1 and MtCEPD2 were activated upon nitrate deficiency in wild-type plants but not cra2 mutant; in contrast to Arabidopsis where AtCEPD1 and AtCEPD2 transcripts were detected only in the shoot, MtCEPD1 and MtCEPD2 transcripts in M. truncatula were identified both in the shoot and root, which suggests that MtCEPD1 and MtCEPD2 may participate in the local regulation of response to nitrate deficiency in the root [50]. In response to rhizobial inoculation, the expression of the MtCEPD1 and MtCEPD2 genes in the shoot did not change and the expression of MtCEPD1 and MtCEPD2 increased in the roots (contrary to expectations of the authors) but this activation did not depend on MtCRA2. Thus, MtCEPD1 and MtCEPD2 do not act as systemic regulators of nodulation in the pathway activated by MtCEP1 and MtCRA2; they probably participate in a local regulation of response to nitrate and rhizobial inoculation within the root. In this work, a possible role of microRNA miR2111 in the MtCEP1-activated signal pathway has been investigated. As it was shown earlier for L. japonicus, miR2111 is a systemic effector, that is translocated from the shoot to root in the absence of rhizobia and mediates degradation of transcripts of the TML gene, an inhibitor of nodulation, thereby making root competent to form symbiosis with rhizobia [49]. Expression of the miR2111 precursor genes revealed by Gautrat et al. in M. truncatula was suppressed in response to rhizobial inoculation and (as it was earlier described for miR2111 in L. japonicus) reduced the amount of the MtTML1/MtTML2 transcripts. Moreover, such regulation was depended on the activity of the SUNN kinase—a key component of AON [50].
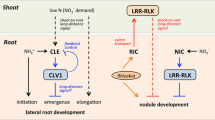
In cra2 mutant, the expression of miR2111 precursor genes was much lower even before inoculation with rhizobia, which was accompanied by an increased content of MtTML1—a putative target of miR2111. miR2111 overexpression resulted in the increase of nodule number in cra2 mutant to the level comparable to the one in wild type. Moreover, the expression of miR2111 precursor genes and the content of mature miR2111 were elevated upon nitrogen deficiency and MtCEP1 overexpression [50]. The obtained results suggest that CEP1 peptide and its putative receptor CRA2 mediate activation of miR2111 expression in the absence of rhizobial inoculation and under low nitrogen in the medium. Grafting experiments have shown that such regulation requires MtCRA2 activity in the shoot. Thus, the level of miR2111, a mobile microRNA transporting from the shoot to the root and stimulating nodulation, is controlled via two regulatory pathways (CEP-CRA2 pathway that positively regulates nodulation, and CLE-SUNN pathway that suppresses nodule development) and also depends on nitrogen content in the soil and on the presence of nitrogen-fixing rhizobial bacteria (Fig. 1).
Participation of CLE and CEP peptides in systemic control of symbiotic nodules development in M. truncatula. Rhizobia release Nod-factors and activate a signal cascade resulting in the formation of primordia of symbiotic nodules. NIN TF activates the expression of CLE genes. CLE peptides (MtCLE12 and MtCLE13) are translocated along the xylem from the root to shoot where they bind in the leaf phloem to CLV1-like kinases (MtSUNN). Tri-arabinosylation of CLE is performed by hydroxyproline-O-arabinosyl transferases encoded by RDN genes. Activation of CLE receptors in the shoot triggers a return signal causing a suppression of nodulation, particulary, through the inhibition of NIN expression. Shoot-derived cytokinin systemically suppresses the formation of nodules. MtSUNN inhibits the expression of the miR2111 genes. miR2111 is a mobile factor inhibiting TML expression, a negative regulator of nodulation. Nitrate suppresses nodule development. Nitrate mediates the accumulation of NLP TF in the nucleus. NLP1 suppresses the action of NIN due to a direct interaction, which probably blocks activation of NIN target genes and results in inhibition of nodulation. Upon nitrate deficiency in the soil, the expression of the CEP genes (MtCEP1) is activated. MtCEP1 is translocated from the root to the shoot where it is recognized in the leaf phloem by a specific receptor, MtCRA2. Activation of MtCRA2 in the shoot stimulates nodulation owing to the suppression of ethylene response in the root. MtCRA2 also stimulates accumulation of miR2111 in the shoot promoting symbiotic nodules development. CEPD genes are induced upon nitrate deficiency in the leaves and roots. CEPD are likely to act locally in the root in M. truncatula. The expression of CEPD is also activated in the roots by rhizobia but their participation in nodulation remains unexplored. Thus, MtCEP1 stimulates and MtCLE12/13 suppress nodulation acting via shoot-acting receptors (MtCRA2 and MtSUNN, respectively).
3. OTHER EXAMPLES OF SECRETED PLANT PEPTIDES CAPABLE OF LONG-DISTANCE TRANSPORT ALONG THE XYLEM
Approximately 1000 genes that putatively encode precursor proteins of regulatory peptides have been identified in Arabidopsis genome [87]. Functions of many of these peptides have not been described so far, and some of them can probably move through the vasculature and mediate communication between different plant organs. Proteomic analysis of xylem sap is a promising approach to detect potentially mobile peptides, but this approach has a number of technical limitations related to a necessity to obtain a sufficient amount of xylem sap for the analysis; until recently, it was only applicable to rather large model plants, such as soybean, maize, and Brassica [88, 89]. Okamoto et al. [90] analysed a peptidome of xylem sap from soybean G. max to identify a potential peptides participating in long-distance transport along the xylem [90]. As a result, seven peptides from 11 to 19 amino acids in length were detected and designated as XAP 1–7 (xylem sap-associated peptides) which undergo one or more posttranslational modification: glycosylation, sulfation, and/or hydroxylation (Table 1). For instance, XAP1 and XAP3 peptides were sulfated at tyrosine residue and abundant in the xylem sap; XAP4 and XAP6 peptides turned out to be homologs of earlier described peptides travelling along the xylem: XAP4 bearing hydroxylated residues of proline and tri-arabinosylated hydroxyproline at position 7 turned out to be a member of CLE peptide family and peptide XAP6 with hydroxylated proline at position 11 is a member of CEP group. In respect to these four peptides (XAP1, XAP3, XAP4, and XAP6), the ability to long-distance transport along the xylem was assessed. Specific mutations were induced in XAP1, XAP3, XAP4, and XAP6 peptides which made it possible to obtain mutant forms of peptides that were distinguishable from native peptides occurring in plants by mass spectrometric characteristics. The mutant forms of XAP1, XAP3, XAP4, and XAP6 peptides produced in transgenic soybean roots obtained as a result of transformation with A. rhizogenes were detected in the shoot xylem sap, which supports their ability to migrate along the xylem from the root to the shoot [90]. Moreover, the authors estimated the the effects of different environmental factors on these peptides in soybean, and it was shown that XAP1 expression was activated upon excess moisture, XAP4 was activated both upon excess moisture and rhizobial inoculation, the expression of the XAP6 gene was suppressed by excess nitrogen and excess moisture, whereas the expression of XAP3 was not significantly changed under the influence of external environmental factors but somewhat decreased upon excess moisture [90].
De Bang et al. [91] later published a work where they identified genes encoding putative regulatory peptides in M. truncatula genome and described almost two thousand genes belonging to 46 different families of regulatory peptides. We have analyzed sequences M. truncatula peptides provided in this paper and found close homologues of XAP1, XAP3, XAP4, and XAP 6 peptides (Table 1).
XAP1 amino acid sequence, shares similarity with peptides of PSY family (PLANT PEPTIDE CONTAINING SULFATED TYROSINE). It is known that a member of this group in Arabidopsis, PSY1 (AtPSY1) peptide stimulates root growth particularly through the activation of proton pumps and apoplast acidification [92]. AtPSY1 interacts with PSY1R (PSY1 RECEPTOR) receptor kinase operating as a heterodimer with SERK1 (SOMATIC EMBRYOGENESIS RECEPTOR KINASE1) whose target is membrane H+-ATPase AHA2 [93]. Interestingly, plant pathogenic bacteria Xanthomonas oryzae also produce sulfated peptides (whose amino acid sequence resembles PSY) capable of simulating growth-producing effect of endogenous PSY peptides [94]. Long-distance transport has not been described for this group of peptides up to date, but taking into account the discovery PSY-like peptide transport through the xylem in soybean, it is interesting to investigate the role of PSY peptides in plant development and long-distance transport as well as in their responses to various external agents.
XAP3 peptide turned out to be identical to a peptide from the CIF (CASPARIAN STRIP INTEGRITY FACTOR) family in M. truncatula. In Arabidopsis, CIF1 and CIF2 peptides regulate Casparian strips development in endodermis [95]. Moreover, the expression of CIF genes in Arabidopsis depends on pH and the concentration of iron salts in the medium, and Arabidopsis cif1-1cif2-1 mutants demonstrate lower resistance to iron salts [95]. Discovery of CIF-like peptide in soybean xylem sap suggests its putative participation in systemic control in plant development in response to exposure to adverse environmental factors.
Interestingly, XAP4 peptide detected in soybean xylem sap turned out to be identical by amino acid sequence to CLE domain of MtCLE42. It was shown earlier that the expression of the MtCLE42 gene is activated upon symbiotic nodule development, but exogenous treatment with peptide MtCLE42 bearing posttranslational modifications did not suppress nodulation in M. truncatula (in contrast to its homologs MtCLE12 and MtCLE13), which suggests that this peptide is not involved in AON [36].
In a study where hairy root culture of M. truncatula was used, a number of secretory peptides present in xylem sap were also identified [96]. As in soybean, in M. truncatula the authors managed to show the presence of secreted peptides of CLE, CEP, CIF, and PSY families in the xylem sap (Table 1). In addition, the biological activity of secreted peptides was assessed in this study. In particular, treatment with exogenous MtCLE17 peptide that was detected in the xylem sap and with MtCLE5 peptide that by amino acid sequence turned out to be identical to CLE domain of TDIF suppressed the growth of the main root and stimulated the formation of lateral roots [96]. However, such an effect was not described earlier for a putative homolog of MtCLE5—peptide TDIF of Arabidopsis encoded by AtCLE41/AtCLE44 genes [97]. Treatment with PSY peptides sulfated at tyrosine and designated by the authors as MtXAP1 and MtXAP5 resulted in a suppression of lateral root development [96].
Thus, the peptidome studies of xylem sap in soybean and M. truncatula identified secreted peptides from CLE and CEP families whose participation in systemic regulation in plants was already known, and the members of two other families, peptides PSY and CIP, whose long-distance transport along the xylem had not been shown before. Detection of PSY and CIP peptides in the xylem sap in two legume species points to a possible participation of these still little-studied peptides in a long-distance transport through the xylem and in systemic control of developmental processes and undoubtedly worth to be investigated in more details.
CONCLUSIONS
Thus, members of two families of peptide regulators (CLE and CEP) are produced in the roots exposed to the influence of different factors, such as availability of water and mineral substances and presence of soil microorganisms; they can travel from the roots to the shoot where specific receptors recognize them in leaf phloem (Fig. 2).
Regulatory peptides and their receptors mediating systemic responses in plants. Peptide AtCLE25 is produced in the roots under drought and moves to the shoot where it binds to its putative receptors AtBAM1/AtBAM3. In response, the expression of the NCED3 gene is activated; this gene is involved in ABA biosynthesis, a hormone that stimulates stomatal closure. Undernitrogen deficiency in the soil, the expression of AtCLE1/3,4,7 is activated in Arabidopsis; their products bind to AtCLV1 receptor that presumably act in the shoot and suppress lateral roots development. Nitrogen deficiency also activates CEPs expression. In A-rabidopsis, AtCEP1 is translocated to the shoot where it binds to AtCEPR receptor; as a result, via CEPD mobile proteins, a return signal stimulating expression of genes encoding nitrate transporters NRT is sent to the root. Under nitrogen deficiency, expression of MtCEP1 is activated in M. truncatula. MtCEP1 is translocated to the shoot where it binds to MtCRA2 receptor and mediates reactions stimulating nodulation on the roots: suppression of ethylene response and activation of miR2111 expression. Moreover, via root-acting MtCRA2 receptor kinase MtCEP1 peptide suppresses lateral root development. A high content of nitrate activates nitrate-regulated CLE in legumes. Products of nitrate-activated LjCLE-RS2,3 genes acting via shoot-acting LjHAR1 CLV1-like kinase, suppress nodulation. Moreover, expression of LjCLE-RS2,3 and LjCLE-RS1 genes is activated upon interaction between plant and rhizobia. Their products travel along the xylem to the leaves where they interact with LjHAR1 in the leaf phloem and trigger responses suppressing subsequent symbiotic nodule formation. In M. truncatula the interaction with rhizobia results in the activation of the MtCLE12,13 genes whose products migrate to the shoot and bind to MtSUNN kinase. MtSUNN indirectly inhibits nodulation via hormones and through a reduction of miR2111 level–a mobile molecule that is transported from the shoot to root and inhibits the expression of TML, an inhibitor of nodulation. In M. truncatula MtCLE53 is induced by mycorrhization and MtCLE33 is a phosphate-activated gene. MtCLE53 and MtCLE33 suppress mycorrhization via shoot-acting MtSUNN receptor kinase. In response, biosynthesis of strigolactones (hormones stimulating development of mycorrhiza) is suppressed. Thus, CLE peptides (MtCLE33 and MtCLE53) and their receptor (MtSUNN) are components of the system of autoregulation of mycorrhization.
The variety of genes encoding CLE and CEP peptides in plants is rather wide; their emergence in the course of evolution apparently promoted the emergence of new regulatory pathways (including systemic regulation) that improved plant adaptability to changing environmental conditions. Interestingly, genes encoding CEP and CLE peptides were found not only in plants but also in the genomes of other organisms that closely interact with them. For instance, in parasitic nematodes causing various outgrowth of plant tissues, the genes encoding CLE and CEP peptides have been identified; their products show biological activity in plant tissues and are even recognized by a host plant receptors mimicking endogenous plant regulators [98, 99]. Moreover, in the genomes of arbuscular mycorrhizal fungi (in four species from the Rhizophagus genus and one species from the Gigaspora genus), genes encoding CLE peptides and affecting the development of host plant root system have been also identified which products are likely to interact with plant receptor complexes [100].
Receptor complexes recognizing CLE and CEP peptides in plants are likely to have a broad specificity as they perceive a number of peptides differing in amino acid sequence, since signaling peptides induced in response to different factors may be perceived by receptor complexes comprising the same proteins. For instance, CLV1-like kinase in M. truncatula, MtSUNN, is assumed to be responsible for the reception of MtCLE12/MtCLE13 peptides activated by rhizobia as well as MtCLE33 and MtCLE53 peptides activated upon phosphate treatment and mycorrhization, respectively (Fig. 2).
The root system in plants has a sensor function reacting to the influence of various stimuli (changes in the level of mineral substances in the soil, its moisture, and the presence of microorganisms) and stimulates the production of regulatory peptides that transfer information about such changes to the shoot. Receptors of such root-derived regulatory transported via xylem are located in leaf phloem and are capable of integrating different signals arriving from the root. Thus, the cells of leaf phloem are a sort of “control centers” that triggers feedback responses and regulates plant developmental processes and plant mineral nutrition on a systemic level. As in the reception of root-derived peptides, the receptor kinases from the total combination of plant leaves are involved, signal shoot-derived signaling molecules transporting to the root reflect a cumulative response of the shoot to the signals received from the root system. Such an exchange of signals between the shoot and root ensured by long-distance transport of regulatory molecules underlies a systemic regulation of plant development and vital functions on the level of the whole organism.
Investigation of molecular bases of this systemic regulation is important from both fundamental and practical standpoints since understanding of the mechanisms of mineral nutrition and influence of macronutrients on plant growth and development will make it possible to formulate new principles of agriculture aiming to optimize the quantity of applied mineral fertilizers sufficient for increasing productivity of important crops.
REFERENCES
Oh, E., Seo, P.J., and Kim, J., Signaling peptides and receptors coordinating plant root development, Trends Plant Sci., 2018, vol. 23, p. 337. https://doi.org/10.1016/j.tplants.2017.12.007
Gancheva, M.S., Malovichko, Yu.V., Poliushkevich, L.O., Dodueva, I.E., and Lutova, L.A., Plant peptide hormones, Russ. J. Plant Physiol., 2019, vol. 66, no. 2, p. 171.
Opsahl-Ferstad, H.G., Le Deunff, E., Dumas, C., and Rogowsky, P.M., ZmEsr, a novel endosperm-specific gene expressed in a restricted region around the maize embryo, Plant J., 1997, vol. 12, p. 235.
Fletcher, J.C., Brand, U., Running, M.P., Simon, R., and Meyerowitz, E.M., Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems, Science, 1999, vol. 283, p. 1911.
Ohyama, K., Ogawa, M., and Matsubayashi, Y., Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis, Plant J., 2008, vol. 55, p. 152. https://doi.org/10.1111/j.1365-313X.2008.03464.x
Delves, A.C., Mathews, A., Day, D.A., Carter, A.S., Carroll, B.J., and Gresshoff, P.M., Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors, Plant Physiol., 1986, vol. 82, p. 588.
Stahl, Y., Wink, R.H., Ingram, G.C., and Simon, R., A signaling module controlling the stem cell niche in Arabidopsis root meristems, Curr. Biol., 2009, vol. 19, p. 909.
Ito, Y., Nakanomyo, I., Motose, H., Iwamoto, K., Sawa, S., Dohmae, N., and Fukuda, H., Dodeca-CLE peptides as suppressors of plant stem cell differentiation, Science, 2006, vol. 313, no. 5788, p. 842.
Vatén, A., Soyars, C.L., Tarr, P.T., Nimchuk, Z.L., and Bergmann, D.C., Modulation of asymmetric division diversity through cytokinin and SPEECHLESS regulatory interactions in the Arabidopsis stomatal lineage, Dev. Cell, 2018, vol. 47, no. 1, p. 53.
Ren, S.C., Song, X.F., Chen, W.Q., Lu, R., Lucas, W.J., and Liu, C.M., CLE25 peptide regulates phloem initiation in Arabidopsis through a CLERK-CLV2 receptor complex, J. Integr. Plant Biol., 2019, vol. 61, p. 1043.
Fiume, E. and Fletcher, J.C., Regulation of Arabidopsis embryo and endosperm development by the polypeptide signaling molecule CLE8, Plant Cell, 2012, vol. 24, p. 1000.
Okamoto, S., Ohnishi, E., Sato, S., Takahashi, H., Nakazono, M., Tabata, S., and Kawaguchi, M., Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation, Plant Cell Physiol., 2009, vol. 50, p. 67.
Mortier, V., Den Herder, G., Whitford, R., van de Velde, W., Rombauts, S., D’Haeseleer, K., Holsters, M., and Goormachtig, S., CLE peptides control Medicago truncatula nodulation locally and systemically, Plant Physiol., 2010, vol. 153, p. 222.
Kiyohara, S. and Sawa, S., CLE signaling systems during plant development and nematode infection, Plant Cell Physiol., 2012, vol. 53, p. 1989.
Oelkers, K., Goffard, N., Weiller, G.F., Gresshoff, P.M., Mathesius, U., and Frickey, T., Bioinformatic analysis of the CLE signaling peptide family, BMC Plant Biol., 2008, vol. 8, p. 1. https://doi.org/10.1186/1471-2229-8-1
Matsubayashi, Y., Post-translationally modified small-peptide signals in plants, Annu. Rev. Plant Biol., 2014, vol. 65, p. 385.
Ohyama, K., Shinohara, H., Ogawa-Ohnishi, M., and Matsubayashi, Y., A glycopeptide regulating stem cell fate in Arabidopsis thaliana,Nat. Chem. Biol., 2009, vol. 8, p. 578.
Hazak, O. and Hardtke, C.S., CLAVATA 1-type receptors in plant development, J. Exp. Bot., 2016, vol. 67, p. 4827.
Yamaguchi, Y.L, Ishida, T., and Sawa, S., CLE peptides and their signaling pathways in plant development, J. Exp. Bot., 2016, vol. 67, p. 4813.
Dolzblasz, A., Nardmann, J., Clerici, E., Causier, B., van der Graaff, E., Chen, J., Davies, B., Werr, W., and Laux, T., Stem cell regulation by Arabidopsis WOX genes, Mol. Plant., 2016, vol. 9, p. 1028.
Dodueva, I.E., Tvorogova, V.E., Azarakhsh, M., Lebedeva, M.A., and Lutova, L.A., Plant stem cells: unity and diversity, Russ. J. Genet. Appl. Res., 2017, vol. 7, no. 4, p. 385. https://doi.org/10.1134/S2079059717040025
Strabala, T.J., O’Donnell, P.J., Smit, A.M., Ampomah-Dwamena, C., Martin, E.J., Netzler, N., Nieuwenhuizen, N.J., Quinn, B.D., Foote, H.C., and Hudson, K.R., Gain-of-function phenotypes of many CLAVATA3/ESR genes, including four new family members, correlate with tandem variations in the conserved CLAVATA3/ESR domain, Plant Physiol., 2006, vol. 140, p. 1331.
Whitford, R., Fernandez, A., De Groodt, R., Ortega, E., and Hilson, P., Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, no. 47, p. 18625.
Clark, S.E., Williams, R.W., and Meyerowitz, E.M., The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis,Cell, 1997, vol. 89, p. 575.
Kinoshita, A., Betsuyaku, S., Osakabe, Y., Mizuno, S., Nagawa, S., Stahl, Y., Simon, R., Yamaguchi-Shinozaki, K., Fukuda, H., and Sawa, S., RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis,Development, 2010, vol. 137, no. 22, p. 3911.
DeYoung, B.J., Bickle, K.L., Schrage, K.J., Muskett, P., Patel, K., and Clark, S.E., The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis,Plant J., 2006, vol. 45, p. 1.
Bleckmann, A., Weidtkamp-Peters, S., Seidel, C., and Simon, R., Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane, Plant Physiol., 2010, vol. 152, p. 166.
Qiang, Y., Wu, J., Han, H., and Wang, G., CLE peptides in vascular development, J. Integr. Plant Biol., 2013, vol. 55, p. 389.
Searle, I.R., Men, A.E., Laniya, T.S., Buzas, D.M., Iturbe-Ormaetxe, I., Carroll, B.J., and Gresshoff, P.M., Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase, Science, 2003, vol. 299, no. 5603, p. 109.
Reid, D.E., Ferguson, B.J., Hayashi, S., Lin, Y.-H., and Gresshoff, P.M., Molecular mechanisms controlling legume autoregulation of nodulation, Ann. Bot., 2011, vol. 789. https://doi.org/10.1093/aob/mcr205
Nishimura, R., Hayashi, M., Wu, G.J., Kouchi, H., Imaizumi-Anraku, H., Murakami, Y., Kawasaki, S., Akao, S., Ohmori, M., Nagasawa, M., Harada, K., and Kawaguchi, M., HAR1 mediates systemic regulation of symbiotic organ development, Nature, 2002, vol. 420, no. 6914, p. 426.
Schnabel, E., Journet, E.P., de Carvalho-Niebel, F., Duc, G., and Frugoli, J., The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length, Plant Mol Biol., 2005, vol. 58, no. 6, p. 809.
Krusell, L., Sato, N., Fukuhara, I., Koch, B.E., Grossmann, C., Okamoto, S., Oka-Kira, E., Otsubo, Y., Aubert, G., Nakagawa, T., Sato, S., Tabata, S., Duc, G., Parniske, M., Wang, T.L., et al., The Clavata2 genes of pea and Lotus japonicus affect autoregulation of nodulation, Plant J., 2011, vol. 65, no. 6, p. 861.
Lim, C.W., Lee, Y.W., and Hwang, C.H., Soybean nodule-enhanced CLE peptides in roots act as signals in GmNARK-mediated nodulation suppression, Plant Cell Physiol., 2011, vol. 52, p. 1613. https://doi.org/10.1093/pcp/pcr091
Samorodova, A.P., Tvorogova, V.E., Tkachenko, A.A., Potsenkovskaya, E.A., Lebedeva, M.A., Tikhonovich, I.A., and Lutova, L.A., Agrobacterial tumors interfere with nodulation and demonstrate the expression of nodulation-induced CLE genes in pea, J. Plant Physiol., 2018, vol. 221, p. 94.
Imin, N., Patel, N., Corcilius, L., Payne, R.J., and Djordjevic, M.A., CLE peptide tri-arabinosylation and peptide domain sequence composition are essential for SUNN-dependent autoregulation of nodulation in Medicago truncatula,New Phytol., 2018, vol. 218, p. 73. https://doi.org/10.1111/nph.15019
Postma, J.G., Jacobsen, E., and Feenstra, W.J., Three pea mutants with an altered nodulation studied by genetic analysis and grafting, J. Plant Physiol., 1988, vol. 132, p. 424. https://doi.org/10.1016/S0176-1617(88)80056-7
Schnabel, E.L., Kassaw, T.K., Smith, L.S., Marsh, J.F., Oldroyd, G.E., Long, S.R., and Frugoli, J.A., The ROOT DETERMINED NODULATION1 gene regulates nodule number in roots of Medicago truncatula and defines a highly conserved, uncharacterized plant gene family, Plant Physiol., 2011, vol. 157, p. 328. https://doi.org/10.1104/pp.111.178756
Kassaw, T., Nowak, S., Schnabel, E., and Frugoli, J., ROOT DETERMINED NODULATION1 is required for M. truncatula CLE12, but not CLE13, peptide signaling through the SUNN receptor kinase, Plant Physiol., 2017, vol. 174, p. 2445. https://doi.org/10.1104/pp.17.00278
Okamoto, S., Shinohara, H., Mori, T., Matsubayashi, Y., and Kawaguchi, M., Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase, Nat. Commun., 2013, vol. 4, p. 2191.
Crook, A.D., Schnabel, E.L., and Frugoli, J.A., The systemic nodule number regulation kinase SUNN in Medicago truncatula interacts with MtCLV2 and MtCR-N, Plant J., 2016, vol. 88, no. 1, p. 108.
Miyazawa, H., Oka-Kira, E., Sato, N., Takahashi, H., Wu, G.-J., Sato, S., Hayashi, M., Betsuyaku, S., Nakazono, M., Tabata, S., Harada, K., Sawa, S., Fukuda, H., and Kawaguchi, M., The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus,Development, 2010, vol. 137, p. 4317. https://doi.org/10.1242/dev.058891
van Noorden, G.E., Ross, J.J., Reid, J.B., Rolfe, B.G., and Mathesius, U., Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant, Plant Physiol., 2006, vol. 140, p. 1494. https://doi.org/10.1104/pp.105.075879
Kinkema, M. and Gresshoff, P.M., Investigation of downstream signals of the soybean autoregulation of nodulation receptor kinase GmNARK, Mol. Plant Micr-obe Interact., 2008, vol. 21, no. 10, p. 1337.
Cheng, C., Li, C., Wang, D., Zhai, L., and Cai, Z., The soybean GmNARK affects ABA and salt responses in transgenic Arabidopsis thaliana,Front. Plant Sci., 2018, vol. 9, p. 514.
Sasaki, T., Suzaki, T., Soyano, T., Kojima, M., Sakakibara, H., and Kawaguchi, M., Shoot-derived cytokinins systemically regulate root nodulation, Nat. Commun., 2014, vol. 5, p. 4983.
Andrade, M.A., González-Guzmán, M., Serrano, R., and Rodríguez, P.L., A combination of the F-box motif and Kelch repeats defines a large Arabidopsis family of F-box proteins, Plant Mol Biol., 2001, vol. 46, no. 5, p. 603.
Takahara, M., Magori, S., Soyano, T., Okamoto, S., Yoshida, C., Yano, K., Sato, S., Tabata, S., Yamaguchi, K., Shigenobu, S., Takeda, N., Suzaki, T., and Kawaguchi, M., TOO MUCH LOVE, a novel Kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume–Rhizobium symbiosis, Plant Cell Physiol., 2013, vol. 54, p. 433. https://doi.org/10.1093/pcp/pct022
Tsikou, D., Yan, Z., Holt, D.B., Abel, N.B., Reid, D.E., Madsen, L.H., Bhasin, H., Sexauer, M., Stougaard, J., and Markmann, K., Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA, Science, 2018, vol. 362, no. 6411, p. 233.
Gautrat, P., Laffont, C., and Frugier, F., Compact root architecture 2 promotes root competence for nodulation through the miR2111 systemic effector, Curr. Biol., 2020, vol. 30, no. 7, p. 1339. https://doi.org/10.1016/j.cub.2020.01.084
Soyano, T., Hirakawa, H., Sato, S., Hayashi, M., and Kawaguchi, M., NODULE INCEPTION creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, p. 14607. https://doi.org/10.1073/pnas.1412716111
Mortier, V., De Wever, E., Vuylsteke, M., Holsters, M., and Goormachtig, S., Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling, Plant J., 2012, vol. 70, p. 367. https://doi.org/10.1111/j.1365-313X.2011.04881.x2012
Nishida, H. and Suzaki, T., Nitrate-mediated control of root nodule symbiosis, Curr. Opin. Plant Biol., 2018, vol. 44, p. 129. https://doi.org/10.1016/j.pbi.2018.04.006
Reid, D.E., Ferguson, B.J., and Gresshoff, P.M., Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation, Mol. Plant Microbe Interact., 2011, vol. 24, p. 606. https://doi.org/10.1094/MPMI-09-10-0207
Lim, C.W., Lee, Y.W., Lee, S.C., and Hwang, C.H., Nitrate inhibits soybean nodulation by regulating expression of CLE genes, Plant Sci., 2014, vol. 229, p. 1. https://doi.org/10.1016/j.plantsci.2014.08.014
Schauser, L., Wieloch, W., and Stougaard, J., Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus,J. Mol. Evol., 2005, vol. 60, no. 2, p. 229.
Konishi, M. and Yanagisawa, S., Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response, Plant J., 2010, vol. 63, no. 2, p. 269.
Nishida, H., Tanaka, S., Handa, Y., Ito, M., Sakamoto, Y., Matsunaga, S., Betsuyaku, S., Miura, K., Soyano, T., Kawaguchi, M., and Suzaki, T., A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus,Nat. Commun., 2018, vol. 499. https://doi.org/10.1038/s41467-018-02831-x
Marchive, C., Roudier, F., Castaings, L., Bréhaut, V., Blondet, E., Colot, V., Meyer, C., and Krapp, A., Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants, Nat. Commun., 2013, vol. 4, p. 1713.
Suzuki, W., Konishi, M., and Yanagisawa, S., The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor, Plant Signaling Behav., 2013, vol. 8, p. e25975. https://doi.org/10.4161/psb.25975
Lin, J.-S., Li, X., Luo, Z., Mysore, K.S., Wen, J., and Xie, F., NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula,Nat. Plants, 2018, vol. 4, p. 942. https://doi.org/10.1038/s41477-018-0261-3
Müller, L.M., Flokova, K., Schnabel, E., Sun, X., Fei, Z., Frugoli, J., Bouwmeester, H.J., and Harrison, M.J., A CLE–SUNN module regulates strigolactone content and fungal colonization in arbuscular mycorrhiza, Nat. Plants, 2019, vol. 5, p. 933. https://doi.org/10.1038/s41477-019-0501-1
Lagunas, B., Achom, M., Bonyadi-Pour, R., Pardal, A.J., Richmond, B.L., Sergaki, C., Vázquez, S., Schäfer, P., Ott, S., Hammond, J., and Gifford, M.L., Regulation of resource partitioning coordinates nitrogen and Rhizobi-a responses and autoregulation of nodulation in Medicago truncatula,Mol. Plant., 2019, vol. 12, p. 833. https://doi.org/10.1016/j.molp.2019.03.014
Araya, T., Miyamoto, M., Wibowo, J., Suzuki, A., Kojima, S., Tsuchiya, Y.N., Sawa, S., Fukuda, H., von Wirén, N., and Takahashi, H., CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, p. 2029. https://doi.org/10.1073/pnas.1319953111
Sun, C.-H., Yu, J.-Q., and Hu, D.-G., Nitrate: a crucial signal during lateral roots development, Front. Plant Sci., 2017, vol. 8. https://doi.org/10.3389/fpls.2017.00485
Olas, J.J., Dingenen, J.V., Abel, C., Działo, M.A., Feil, R., Krapp, A., Schlereth, A., and Wahl, V., Nitrate acts at the Arabidopsis thaliana shoot apical meristem to regulate flowering time, New Phytol., 2019, vol. 223, p. 814. https://doi.org/10.1111/nph.15812
Wopereis, J., Pajuelo, E., Dazzo, F.B., Jiang, Q., Gresshoff, P.M., De Bruijn, F.J., Stougaard, J., and Szczyglowski, K., Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype, Plant J., 2000, vol. 23, p. 97. https://doi.org/10.1046/j.1365-313x.2000.00799.x
Liu, K.H., Niu, Y., Konishi, M., Wu, Y., Du, H., Sun Chung, H., Li, L., Boudsocq, M., McCormack, M., Maekawa, S., Ishida, T., Zhang, C., Shokat, K., Yanagisawa, S., and Sheen, J., Discovery of nitrate-CPK-NLP signaling in central nutrient-growth networks, Nature, 2017, vol. 545, p. 311.
Guan, P., Ripoll, J.-J., Wang, R., Vuong, L., Bailey-Steinitz, L.J., Ye, D., and Crawford, N.M., Interacting TCP and NLP transcription factors control plant responses to nitrate availability, Proc. Natl. Acad. Sci. U.S.A., 2017, vol. 114, p. 2419. https://doi.org/10.1073/pnas.1615676114
Hirose, N., Takei, K., Kuroha, T., Kamada-Nobusada, T., Hayashi, H., and Sakakibara, H., Regulation of cytokinin biosynthesis, compartmentalization and translocation, J. Exp. Bot., 2008, vol. 59, p. 75.
Landrein, B., Formosa-Jordan, P., Malivert, A., Schuster, C., Melnyk, C.W., Yang, W., Turnbull, C., Meyerowitz, E.M., Locke, J.C.W., and Jönsson, H., Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins, Proc. Natl. Acad. Sci. U.S.A., 2018, vol. 115, p. 1382. https://doi.org/10.1073/pnas.1718670115
Takahashi, F., Suzuki, T., Osakabe, Y., Betsuyaku, S., Kondo, Y., Dohmae, N., Fukuda, H., Yamaguchi-Shinozaki, K., and Shinozaki, K., A small peptide modulates stomatal control via abscisic acid in long-distance signaling, Nature, 2018, vol. 556, p. 235. https://doi.org/10.1038/s41586-018-0009-2
Ren, S.-C., Song, X.-F., Chen, W.-Q., Lu, R., Lucas, W.J., and Liu, C.-M., CLE25 peptide regulates phloem initiation in Arabidopsis through a CLERK-CLV2 receptor complex, J. Integr. Plant Biol., 2019, vol. 61, p. 1043. https://doi.org/10.1111/jipb.12846
Goad, D.M., Zhu, C., and Kellogg, E.A., Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function, New Phytol., 2017, vol. 2, p. 605.
Ogilvie, H.A., Imin, N., and Djordjevic, M.A., Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes, BMC Genomics, 2014, vol. 15, p. 870.
Delay, C., Chapman, K., Taleski, M., Wang, Y., Tyagi, S., Xiong, Y., Imin, N., and Djordjevic, M.A., CEP3 levels affect starvation-related growth responses of the primary root, J. Exp. Bot., 2019, vol. 70, no. 18, p. 4763-4774.
Tabata, R., Sumida, K., Yoshii, T., Ohyama, K., Shinohara, H., and Matsubayashi, Y., Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling, Science, 2014, vol. 346, no. 6207, p. 343.
Ruffel, S., Krouk, G., Ristova, D., Shasha, D., Birnbaum, K.D., and Coruzzi, G.M., Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand, Proc. Natl. Acad. Sci. U.S.A., 2011, vol. 108, no. 45, p. 18524.
Ohkubo, Y., Tanaka, M., Tabata, R., Ogawa-Ohnishi, M., and Matsubayashi, Y., Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition, Nat. Plants, 2017, vol. 3, p. 1. https://doi.org/10.1038/nplants.2017.29
Ota, R., Ohkubo, Y., Yamashita, Y., Ogawa-Ohnishi, M., and Matsubayashi, Y., Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis,Nat. Commun., 2020, vol. 11, no. 1, p. 641.
Roberts, I., Smith, S., Stes, E., De Rybel, B., Staes, A., van de Cotte, B., Njo, M.F., Dedeyne, L., Demol, H., Lavenus, J., Audenaert, D., Gevaert, K., Beeckman, T., and De Smet, I., CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis,J. Exp. Bot., 2016, vol. 67, no. 16, p. 4889.
Mohd-Radzman, N.A., Laffont, C., Ivanovici, A., Patel, N., Reid, D., Stougaard, J., Frugier, F., Imin, N., and Djordjevic, M.A., Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development, Plant Physiol., 2016, vol. 171, no. 2536. https://doi.org/10.1104/pp.16.00113
Imin, N., Mohd-Radzman, N.A., Ogilvie, H.A., and Djordjevic, M.A., The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula,J. Exp. Bot., 2013, vol. 64, p. 5395. https://doi.org/10.1093/jxb/ert369
Huault, E., Laffont, C., Wen, J., Mysore, K.S., Ratet, P., Duc, G., and Frugier, F., Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase, PLoS Genet., 2014, vol. 10, no. 12, p. e1004891.
Ju, C. and Chang, C., Mechanistic insights in ethylene perception and signal transduction, Plant Physiol., 2015, vol. 169, no. 1, p. 85.
Penmetsa, R.V., Uribe, P., Anderson, J., Lichtenzveig, J., Gish, J.C., Nam, Y.W., Engstrom, E., Xu, K., Sckisel, G., Pereira, M., Baek, J.M., Lopez-Meyer, M., Long, S.R., Harrison, M.J., Singh, K.B., et al., The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations, Plant J., 2008, vol. 55, no. 4, p. 580.
Lease, K.A. and Walker, J.C., The Arabidopsis unannotated secreted peptide database, a resource for plant peptidomics, Plant Physiol., 2006, vol. 142, no. 3, p. 831.
Kehr, J., Buhtz, A., and Giavalisco, P., Analysis of xylem sap proteins from Brassica napus,BMC Plant Biol., 2005, vol. 5, p. 11.
Alvarez, S., Marsh, E.L., Schroeder, S.G., and Schachtman, D.P., Metabolomic and proteomic changes in the xylem sap of maize under drought, Plant Cell Environ., 2008, vol. 31, no. 3, p. 325.
Okamoto, S., Suzuki, T., Kawaguchi, M., Higashiyama, T., and Matsubayashi, Y., A comprehensive strategy for identifying long-distance mobile peptides in xylem sap, Plant J., 2015, vol. 84, p. 611. https://doi.org/10.1111/tpj.13015
de Bang, T.C., Lundquist, P.K., Dai, X., Boschiero, C., Zhuang, Z., Pant, P., Torres-Jerez, I., Roy, S., Nogales, J., Veerappan, V., Dickstein, R., Udvardi, M.K., Zhao, P.X., and Scheible, W.-R., Genome-wide identification of Medicago peptides involved in macronutrient responses and nodulation, Plant Physiol., 2017, vol. 175, p. 1669.
Amano, Y., Tsubouchi, H., Shinohara, H., Ogawa, M., and Matsubayashi, Y., Tyrosine-sulfated glycopeptide involved in cellular proliferation and expansion in Arabid-opsis,Proc. Natl. Acad. Sci. U.S.A., 2007, vol. 104, no. 46, p. 18333.
Fuglsang, A.T., Kristensen, A., Cuin, T.A., Schulze, W.X., Persson, J., Thuesen, K.H., Ytting, C.K., Oehlenschlæger, C.B., Mahmood, K., Sondergaard, T.E., Shabala, S., and Palmgren, M.G., Receptor kinase-mediated control of primary active proton pumping at the plasma membrane, Plant J., 2014, vol. 80, p. 951.
Pruitt, R.N., Joe, A., Zhang, W., Feng, W., Stewart, V., Schwessinger, B., Dinneny, J.R., and Ronald, P.C., A microbially derived tyrosine-sulfated peptide mimics a plant peptide hormone, New Phytol., 2017, vol. 215, no. 2, p. 725.
Nakayama, T., Shinohara, H., Tanaka, M., Baba, K., Ogawa-Ohnishi, M., and Matsubayashi, Y., A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots, Science, 2017, vol. 355, no. 6322, p. 284.
Patel, N., Mohd-Radzman, N.A., Corcilius, L., Crossett, B., Connolly, A., Cordwell, S.J., Ivanovici, A., Taylor, K., Williams, J., Binos, S., Mariani, M., Payne, R.J., and Djordjevic, M.A., Diverse peptide hormones affecting root growth identified in the Medic-ago truncatula secreted peptidome, Mol. Cell. Proteomics, 2018, vol. 17, p. 160. https://doi.org/10.1074/mcp.RA117.000168
Hirakawa, Y., Shinohara, H., Welke, K., Irle, S., Matsubayashi, Y., Torii, K.U., and Uchida, N., Cryptic bioactivity capacitated by synthetic hybrid plant peptides, Nat. Commun., 2017, vol. 8, p. 14318.
Wang, X., Mitchum, M.G., Gao, B., Li, C., Diab, H., Baum, T.J., Hussey, R.S., and Davis, E.L., A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR (CLE) of Arabidopsis thaliana,Mol. Plant Pathol., 2005, vol. 6, no. 2, p. 187.
Eves-Van Den Akker, S., Lilley, C.J., Yusup, H.B., Jones, J.T., and Urwin, P.E., Functional C-TERMINALLY ENCODED PEPTIDE (CEP) plant hormone domains evolved de novo in the plant parasite Rotylenchulus reniformis,Mol. Plant Pathol., 2016, vol. 17, no. 8, p. 1265.
Le Marquer, M., Bécard, G., and Frei Dit Frey, N., Arbuscular mycorrhizal fungi possess a CLAVATA3/embryo surrounding region-related gene that positively regulates symbiosis, New Phytol., 2019, vol. 222, no. 2, p. 1030.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 19-116-50095 (Expansion).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies involving animals or human participants performed by any of the authors. The authors declare that they have no conflict of interest.
Additional information
Translated by N. Balakshina
Abbreviations: AON—autoregulation of nodulation; PTMP—posttranslationally modified peptides; RAM—root apical meristem; SAM—shoot apical meristem; TF—transcription factor
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4. International license (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lebedeva, M.A., Yashenkova, Y.S., Dodueva, I.E. et al. Molecular Dialog between Root and Shoot via Regulatory Peptides and Its Role in Systemic Control of Plant Development. Russ J Plant Physiol 67, 985–1002 (2020). https://doi.org/10.1134/S1021443720060114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720060114