Abstract
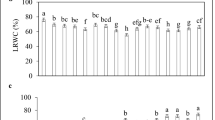
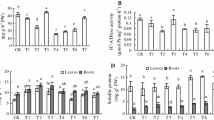
We investigated effects of sodium nitroprusside (SNP), the donor of nitric oxide (NO), on the growth and hormonal system of wheat plants (Triticum aestivum L.) in normal conditions and after salt stress (2% NaCl). During germination of seeds treated with SNP (50–500 μM), we obtained the SNP concentration (200 μM) optimal for stimulation of seedling growth estimated by increase in seed germination capacity and seedlings' linear sizes and their fresh and dry biomass. A comparative analysis of SNP (200 μM) effects, after seed germination in the medium with SNP or pretreatment of 3-day-old seedlings, showed SNP ability to increase the wheat plant resistance to subsequent effects of sodium chloride salinity at both treatment methods. Protective SNP effects appeared in the reduction of stress inhibitory action on seedling growth rates and significant reduction in the level of lipid peroxidation and exosmosis of electrolytes. An important contribution to realization of the growth-stimulating and protective effects of NO is associated with its ability to influence the state of the hormonal system of wheat plants due to an increase in the concentration of hormones of a cytokinin nature under normal conditions and the prevention of a decrease in their level under stress.
Similar content being viewed by others
Abbreviations
- SNP:
-
sodium nitroprusside
References
Krasylenko, Yu.A., Yemets, A.I., and Blume, Ya.B., Functional role of nitric oxide in plants, Russ. J. Plant Physiol., 2010, vol. 57, pp. 451–461.
Sanz, L., Albertos, P., Mateos, I., Sanchez-Vicente, I., Fernandez-Marcos, M., and Lorenzo, O., Nitric oxide (NO) and phytohormones crosstalk during early plant development, J. Exp. Bot., 2015, vol. 66, pp. 2857–2868.
Mamaeva, A.S., Fomenkov, A.A., Nosov, A.V., Moshkov, I.E., Mur, L.A.J., Hall, M.A., and Novikova, G.V., Regulatory role of nitric oxide in plants, Russ. J. Plant Physiol., 2015, vol. 62, pp. 427–440.
Šírová, J., Sedlářová, M., Piterková, J., Luhová, L., and Petřivalský, M., The role of nitric oxide in the germination of plant seeds and pollen, Plant Sci., 2011, vol. 181, pp. 560–572.
Pagnussat, G.C., Simontachii, M., Puntarulo, S., and Lamattina, L., Nitric oxide is required for root organogenesis, Plant Physiol., 2002, vol. 129, pp. 954–956.
Hu, X., Neill, S.J., Tang, Z., and Cai, W., Nitric oxide mediates gravitropic bending in soybean roots, Plant Physiol., 2005, vol. 137, pp. 663–670.
Neill, S., Barroso, R., Bright, J., Desikan, R., Hancock, J., Harrison, J., Morris, P., Ribeiro, D., and Wilson, J., Nitric oxide, stomatal closure, and abiotic stress, J. Exp. Bot., 2008, vol. 59, pp. 165–176.
Zhou, Y., Zhang, Y., Wang, X., Cui, J., Xia, X., Shi, K., and Yu, J., Effects of nitrogen form on growth, CO2 assimilation, chlorophyll fluorescence, and photosynthetic electron allocation in cucumber and rice plants, J. Zhejiang Univ., Sci. B., 2011, vol. 12, pp. 126–134.
Domingos, P., Prado, A.M., Wong, A., Gehring, Ch., and Feijo, J.A., Nitric oxide: a multitasked signaling gas in plants, Mol. Plant, 2015, vol. 8, pp. 506–520.
Manjunatha, G., Lokesh, V., and Neelwarne, B., Nitric oxide in fruit ripening: trends and opportunities, Biotechnol. Adv., 2010, vol. 28, pp. 489–499.
Mur, L.A.J., Prats, E., Pierre, S., Hall, M., and Hebelstrup, K.H., Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways, Front. Plant Sci., 2013, vol. 4, p. 215. doi 10.3389/ fpls.2013.00215
Tian, X. and Lei, Y., Nitric oxide treatment alleviates drought stress in wheat seedlings, Biol. Plant., 2006, vol. 50, pp. 775–778.
Boyarshinov, A.V. and Asafova, E.V., Stress responses of wheat leaves to dehydration: participation of endogenous NO and effect of sodium nitroprusside, Russ. J. Plant Physiol., 2011, vol. 58, pp. 1034–1039.
Zheng, Ch., Jiang, D., Liu, F., Dai, T., Liu, W., Jing, Q., and Cao, W., Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity, Environ. Exp. Bot., 2009, vol. 67, pp. 222–227.
Dong, Y.J., Jinc, S.S., Liu, S., Xu, L.L., and Kong, J., Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress, J. Soil Sci. Plant Nutr., 2014, vol. 14, pp. 1–13. doi 10.4067/s0718-95162014005000001
Gil'vanova, I.R., Enikeev, A.R., Stepanov, S.Yu., and Rakhmankulova, Z.F., Involvement of salicylic acid and nitric oxide in protective reactions of wheat under the influence of heavy metals, Appl. Biochem. Mikrobiol., 2012, vol. 48, no. 1, pp. 90–94.
Sun, Ch., Lu, L., Liu, L., Liu, W., Yu, Y., Liu, X., Hu, Y., Jin, Ch., and Lin, X., Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum), New Phytol., 2014, vol. 201, pp. 1240–1250.
Zhao, M., Chen, L., Zhang, L., and Zhang, W., Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis, Plant Physiol., 2009, vol. 151, pp. 755–767.
Karpets, Yu.V., Kolupaev, Yu.E., and Vayner, A.A., Functional interaction between nitric oxide and hydrogen peroxide during formation of wheat seedling induced heat resistance, Russ. J. Plant Physiol., 2015, vol. 62, pp. 65–70.
Tian, X.R. and Lei, Y.B., Physiological responses of wheat seedlings to drought and UV-B radiation. Effect of exogenous sodium nitroprusside application, Russ. J. Plant Physiol., 2007, vol. 54, pp. 676–682.
Shakirova, F.M., Bezrukova, M.V., Fatkhutdinova, R.A., Sakhabutdinova, A.R., and Fatkhutdinova, D.R., Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity, Plant Sci., 2003, vol. 164, pp. 317–322.
Bezrukova, M., Kildibekova, A., and Shakirova, F., WGA reduces the level of oxidative stress in wheat seedlings under salinity, Plant Growth Regul., 2008, vol. 54, pp. 195–201.
Shakirova, F., Avalbaev, A., Bezrukova, M., Fatkhutdinova, R., Maslennikova, D., Yuldashev, R., Allagulova, C., and Lastochkina, O., Hormonal intermediates in the protective action of exogenous phytohormones in wheat plants under salinity, in Phytohormones and Abiotic Stress Tolerance in Plants, Khan, N., Nazar, R., Iqbal, N., and Anjum, N., Eds., Berlin: Springer-Verlag, 2012, pp. 185–228.
Ryu, H. and Cho, Y.G., Plant hormones in salt stress tolerance, J. Plant Biol., 2015, vol. 58, pp. 147–155.
Freschi, L., Nitric oxide and phytohormone interaction: current status and perspectives, Front. Plant Sci., 2013, vol. 4, p. 398. doi 10.3389/fpls.2013.00398
Li, X., Pan, Y., Chang, B., Wang, Y., and Tang, Z., NO promotes seed germination and seedling growth under high salt may depend on EIN3 protein in Arabidopsis, Front. Plant Sci., 2016, vol. 6, p. 1203. doi 10.3389/ fpls.2015.01203
Argueso, C.T., Raines, T., and Kieber, J.J., Cytokinin signaling and transcriptional networks, Curr. Opin. Plant Biol., 2010, vol. 13, pp. 533–539.
Ha, S., Vankova, R., Yamaguchi-Shinozaki, K., Shinozaki, K., and Tran, L.S.Ph., Cytokinins: metabolism and function in plant adaptation to environmental stresses, Trends Plant Sci., 2012, vol. 17, pp. 172–179.
Alavi S.M.N., Arvin M.J., and Kalantari, K.M., Salicylic acid and nitric oxide alleviate osmotic stress in wheat (Triticum aestivum L.) seedlings, J. Plant Interact., 2014, vol. 9, pp. 683–688.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.R. Maslennikova, Ch.R. Allagulova, K.A. Fedorova, A.A. Plotnikov, A.M. Avalbaev, F.M. Shakirova, 2017, published in Fiziologiya Rastenii, 2017, Vol. 64, No. 5, pp. 355–362.
Rights and permissions
About this article
Cite this article
Maslennikova, D.R., Allagulova, C.R., Fedorova, K.A. et al. Cytokinins contribute to realization of nitric oxide growth-stimulating and protective effects on wheat plants. Russ J Plant Physiol 64, 665–671 (2017). https://doi.org/10.1134/S1021443717040094
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443717040094