Abstract
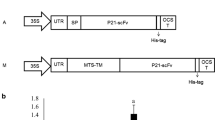
The acidic fibroblast growth factor (aFGF) is a member of FGF family which is composed of 154 amino acids. In order to explore the functional anti-aFGF single-chain variable fragment antibody (scFv) expression in eukaryote system and to enhance the binding activity, the functional scFv specific for human aFGF was expressed in tobacco plants via TMV vector mediated by Agrobacterium. The engineered bacterial EHA105: p35S-30B-scFv was inoculated into the leaves of Nicotiana benthamiana plants by needleless injection. Green fluorescence protein was used as a reporter gene and accumulated at the highest level on the 8th day of post-inoculation. Expression of scFv was identified by RT-PCR and Western-blot. The yield of anti-aFGF-scFv protein was reached to 0.5% of the plant total soluble proteins and a fair binding activity with its antigen aFGF was detected.
Similar content being viewed by others
References
Vallés, A.M., Boyer, B., Badet, J., Tucker, G.C., Barritault, D., and Thiery, J.P., Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line, Proc. Natl. Acad. Sci. USA, 1990, vol. 87, pp. 124–128.
Yamanaka, Y., Over expression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage 1, Cancer Res., 1993, vol. 53, pp. 5289–5296.
Chow, N.H., Cheng, K.S., Lin, P.W., Chan, S.H., Su, W.C., Sun, Y.N., and Lin, X.Z., Expression of fibroblast growth factor-1 and fibroblast growth factor-2 in normal liver and hepatocellular carcinoma, Dig. Dis. Sci., 1998, vol. 43, pp. 2261–2266.
Chames, P. and Baty, D., Antibody engineering and its application in tumor targeting and intracellular immunization, FEMS Microbiol. Lett., 2000, vol. 189, pp. 1–8.
Colcher, D., Bird, R., Roselli, M., Hardman, K.D., Johnson, S., Pope, S., Dodd, S.W., Pantoliano, M.W., Milenic, D.E., and Schlom, J., In vivo tumor targeting of a recombinant single-chain antigen-binding protein, J. Natl. Cancer Inst., 1990, vol. 82, pp. 1191–1197.
Yokota, T., Milenic, D.E., Whitlow, M., and Schlom, J., Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms, Cancer Res., 1992, vol. 52, pp. 3402–3408.
Arun, K. and Sharma, M.K., Plants as bioreactors: recent developments and emerging opportunities, Biotechnol. Adv., 2009, vol. 27, pp. 811–832.
Lau, O.S. and Sun, S.S., Plant seeds as bioreactors for recombinant protein production, Biotechnol. Adv., 2009, vol. 27, pp. 1015–1022.
Sun, Q.Y., Ding, L.W., Lomonossoff, G.P., Sun, Y.B., Luo, M., Li, C.Q., Jiang, L., and Xu, Z.F., Improved expression and purification of recombinant human serum albumin from transgenic tobacco suspension culture, J. Biotechnol., 2011, vol. 155, pp. 164–172.
De Zoeten, G., Penswick, J.R., and Horisberger, M.A., The expression, localization, and effect of a human interferon in plants, Virology, 1989, vol. 172, pp. 213–222.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Hiatt, A., Cafferkey, R., and Bowdish, K., Production of antibodies in transgenic plants, Nature, 1989, vol. 342, pp. 76–78.
Fiedler, U., Phillips, J., Artsaenko, O., and Conrad, U., Optimization of scFv antibody production in transgenic plants, Immunotechnology, 1997, vol. 3, pp. 205–216.
Artsaenko, O., Peisker, M., zur Nieden, U., Fiedler, U., Weiler, E.W., Müntz, K., and Conrad, U., Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco, Plant J., 1995, vol. 8, pp. 745–750.
Lindbo, J.A., High-efficiency protein expression in plants from agroinfection-compatible Tobacco mosaic virus expression vectors, BMC Biotechnology, 2007, vol. 7: 52.
Turpen, T.H., Turpen, A.M., Weinzettl, N., Kumagai, M.H., and Dawson, W., Transfection of whole plants from wounds inoculated with Agrobacterium tumefaciens containing cDNA of tobacco mosaic virus, J. Virol. Methods, 1993, vol. 42, pp. 227–239.
Shivprasad, S., Pogue, G.P., Lewandowski, D.J., Hidalgo, J., Donson, J., Grill, L.K., and Dawson, W., Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vector, Virology, 1999, vol. 255, pp. 312–323.
Marillonnet, S., Thoeringer, C., Kandzia, R., Klimyuk, V., and Gleba, Y., Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants, Nat. Biotechnol., 2005, vol. 23, pp. 718–723.
Schubbert, R., Hohlweg, U., and Renz, D., On the fate of orally ingested foreign DNA in mice: chromosomal association and placental transmission to the fetus, Mol. Gen. Genet., 1998, vol. 259, pp. 569–576.
Wandelt, C.I., Khan, M.R., Craig, S., Schroeder, H.E., Spencer, D., and Higgins, T.J., Vicilin with carboxyterminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants, Plant J., 1992, vol. 2, pp. 181–192.
Ko, K. and Koprowski, H., Plant biopharming of monoclonal antibodies, Virus Res., 2005, vol. 111, pp. 93–100.
Author information
Authors and Affiliations
Corresponding author
Additional information
This text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Wang, X.F., Li, L., Yang, T. et al. Single-chain variable fragment (scFv) expression in tobacco plants via agroinoculation. Russ J Plant Physiol 62, 401–407 (2015). https://doi.org/10.1134/S102144371503019X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S102144371503019X