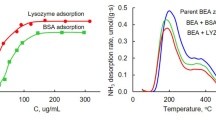
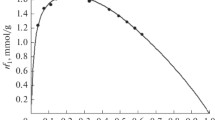
Abstract
The kinetics of the adsorption of lysozyme and bovine serum albumin (BSA) onto zeolites of BEA and MFI structural types and onto silica adsorbents of the same structural types was studied. The rate constants of the reversible adsorption step were calculated. The adsorption rate constants are 0.4–0.8 L mol–1 s–1 for lysozyme and 0.7–1.4 L mol–1 s–1 for BSA. The rate constants of the desorption from the surface of all the samples are in the range from 1.3 × 10–5 to 1.6 × 10–5 s–1 for both enzymes.





Similar content being viewed by others
REFERENCES
Chukhrai, E.S. and Atyaksheva, L.F., Adsorption of proteins on silicas, Fizicheskaya khimiya bioprotsessov (Physical Chemistry of Biological Processes), Varfolomeev, S.D., Ed., Moscow: KRASAND, 2014, pp. 337–380.
Zhang, H., Jiang, Z., Xia, Q., and Zhou, D., Biochem. Eng. J., 2021, vol. 172, article 108033. https://doi.org/10.1016/j.bej.2021.108033
Carlsson, N., Gustafsson, H., Thӧrn, C., Olsson, L., Holmberg, K., and Åkerman, B., Adv. Colloid Interface Sci., 2014, vol. 205, pp. 339–360. https://doi.org/10.1016/j.cis.2013.08.010
Welsch, N., Lu, Y., Dzubiella, J., and Ballauff, M., Polymers, 2013, vol. 54, pp. 2835–2849. https://doi.org/10.1016/j.polymer.2013.03.027
Hu, Z., Chen, Z., Chen, X., and Wang, J., TrAC, 2022, vol. 153, article 116627. https://doi.org/10.1016/j.trac.2022.116627
Soderquist, M.E. and Walton, A.G., J. Colloid Interface Sci., 1980, vol. 75, no. 2, pp. 386–397.
Poltorak, O.M., Pryakhin, A.N., and Chukhrai, E.S., Vestn. Mosk. Univ., Ser. 2: Khimiya, 1982, vol. 23, no. 6, pp. 527–543.
Van Tassel, P.R., Viot, P., and Tarjus, G., J. Chem. Phys., 1997, vol. 106, pp. 761–770. https://doi.org/10.1063/1.473164
Latour, R.A., Colloids Surf. B, 2020, vol. 191, article 110992. https://doi.org/10.1016/j.colsurfb.2020.110992
Vu, T.N., Le, P.H.P., Truong, T.T.T., Nguyen, P.T., Dinh, T.D., Tran, T.K., Hoang, T.H., and Pham, T.D., J. Mol. Liq., 2023, vol. 121, article 121903. https://doi.org/10.1016/j.molliq.2023.121903
Shaha, M.T. and Alveroglu, E., Mater. Sci. Eng. C, 2017, vol. 81, pp. 393–399. https://doi.org/10.1016/j.msec.2017.08.033
Chang, Yu-K., Chu, L., Tsai, J.-C., and Chiu, S.-J., Process Biochem., 2006, vol. 41, pp. 1864–1874. https://doi.org/10.1016/j.procbio.2006.03.039
Cheng, T.-H., Sankaran, R., Show, P.L., Ooi, C.W., Liu, B-L., Chai, W.S., and Chang, Y-K., Int. J. Biol. Macromol., 2021, vol. 185, pp. 761–772. https://doi.org/10.1016/j.ijbiomac.2021.06.177
Hwang, M.-J., Kim, O.-H., Shim, W.-G., and Moon, H., Micropor. Mesopor. Mater., 2013, vol. 182, pp. 81–86. https://doi.org/10.1016/j.micromeso.2013.08.022
Bazzaz, F., Binaeian, E., Heydarinasab, A., and Arezou Ghadi, A., Adv. Powder Technol., 2018, vol. 29, pp. 1664–1675. https://doi.org/10.1016/j.apt.2018.04.001
Flanigen, E.M., Bennet, J.M., Crose, R.W., Cohen, J.P., Patton, R.L., Kircher, R.M., and Smith, J.V., Nature, 1978, vol. 271, pp. 512–516.
Atyaksheva, L.F., Dobryakova, I.V., Kostyukov, I.A., and Kolyagin, Yu.G., Petrol. Chem., 2022, vol. 62, no. 3, pp. 316–321. https://doi.org/10.1134/S0965544122030021
Matsui, M., Kiyozumi, Y., Mizushina, Y., Sakaguchi, K., and Mizukami, F., Sep. Purif. Technol., 2015, vol. 149, pp. 103–109. https://doi.org/10.1016/j.seppur.2015.05.023
Steri, D., Monduzzi, M., and Salis, A., Micropor. Mesopor. Mater., 2013, vol. 170, pp. 164–172. https://doi.org/10.1016/j.micromeso.2012.12.002
Swain, S.K. and Sarkar, D., Appl. Surf. Sci., 2013, vol. 286, pp. 99–103. https://doi.org/10.1016/j.apsusc.2013.09.027
Lee, S.Y., Show, P.L., Ko, C.-M., and Chang, Y.-K., Biochem. Eng. J., 2019, vol. 141, pp. 210–216. https://doi.org/10.1016/j.bej.2018.10.016
Avery, K.L., Peixoto, C., Barcellona, M., Bernards, M.T., and Hunt, H.K., Mater. Today Commun., 2019, vol. 19, pp. 352–359. https://doi.org/10.1016/j.mtcomm.2019.03.004
Daly, S.M., Przybycien, T.M., and Tilton, R.D., Colloids Surf. B, 2007, vol. 57, pp. 81–88. https://doi.org/10.1016/j.colsurfb.2007.01.007
Carter, D.C. and Ho, J.X., Adv. Protein Chem., 1994, vol. 45, pp. 153–203. https://doi.org/10.1016/S0065-3233(08)60640-3
Jachimska, B., Tokarczyk, K., Łapczyńska, M., PuciulMalinowska, A., and Zapotoczny, S., Colloids Surf. A, 2016, vol. 489, pp. 163–172. https://doi.org/10.1016/j.colsurfa.2015.10.033
Atyaksheva, L.F., Pilipenko, O.S., and Tarasevich, B.N., Russ. J. Phys. Chem. A, 2021, vol. 95, pp. 188–192. https://doi.org/10.1134/S0036024421010039
Funding
The synthesis of BEA zeolites, study of their physicochemical and acid properties, and study of the lysozyme adsorption kinetics were financially supported by the Russian Science Foundation (project no. 20-13-00203, https://rscf.ru/project/20-13-00203).
The kinetics of the BSA adsorption onto the surface of ZSM-5 zeolite and silicalite-1 was studied within the framework of the government assignment: Physical Chemistry of the Surface, Adsorption, and Catalysis.
Studies of the phase composition and morphology of the samples were financially supported by the Science and Universities national project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atyaksheva, L.F., Dobryakova, I.V., Enbaev, Z.S. et al. Kinetic Relationships of the Adsorption of Lysozyme and Bovine Serum Albumin onto Zeolites of BEA and MFI Structural Types. Pet. Chem. (2024). https://doi.org/10.1134/S0965544124010110
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0965544124010110