Abstract
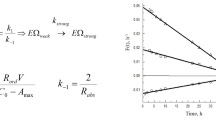
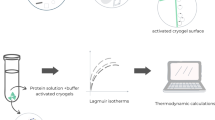
A modified Poisson–Boltzmann model (PBEm) can be successfully used to determine the binding strength parameter, i.e., (Henry constant, K), for the protein adsorbent interaction in ion-exchanger columns. Lysozyme has been employed as a standard protein for the adsorption in a mesoporous silica adsorbent. The density of aminoacid groups and silanol groups were used as inputs to calculate the protein charge density as a function of pH, salt concentration, and type of salt. Using the electrostatic potential provided as solving the PBEm with the protein charge surface and silanol wall as boundaries conditions, we calculated the K through the potential of mean force to describe the whole set of experimental data. The unique estimated parameter in this work was the volumetric accessible surface area from isotherm data for different electrolyte conditions. The results show that the protocol applied includes a pH and ionic strength dependence in the Langmuir isotherm. A sensibility test with different anions (\(\text {Cl}^{-}\), \(\text {Br}^{-}\), and \(\text {I}^{-}\)) showed an agreement with the Hofmeister series for the protein/adsorbent interaction. A modification in the electrolyte concentration and pH can change the behavior of the isotherm profile for a fixed value of saturation capacity, independently calculated for spheres packed in cylinders. The calculations provide here can be helpful for the optimization of the best condition for protein adsorption.









Similar content being viewed by others

References
Santos, S.M.L.D., Nogueira, K.A.B., Gama, M.D.S., Lima, J.D.F., Silva Júnior, L.J.D., Azevedo, D.C.S.D.: Synthesis and characterization of ordered mesoporous silica (sba-15 and sba-16) for adsorption of biomolecules. Microporous Mesoporous Mater. 180, 284–292 (2013). https://doi.org/10.1016/j.micromeso.2013.06.043
Regnier, F.: High-performance liquid chromatography of biopolymers. Science 222(4621), 245–252 (1983). https://doi.org/10.1126/science.6353575
Regnier, F.E., Mazsaroff, I.: A theoretical examination of adsorption processes in preparative liquid chromatography of proteins. Biotechnol. Prog. 3(1), 22–26 (1987). https://doi.org/10.1002/btpr.5420030105
Brooks, C.A., Cramer, S.M.: Steric mass-action ion exchange: Displacement profiles and induced salt gradients. AIChE J. 38(12), 1969–1978 (1992). https://doi.org/10.1002/aic.690381212
Iyer, H., Tapper, S., Lester, P., Wolk, B., van Reis, R.: Use of the steric mass action model in ion-exchange chromatographic process development. J. Chromatogr. A 832(1), 1–9 (1999). https://doi.org/10.1016/S0021-9673(98)01002-4
Geng, X., Zebolsky, D.M.: The stoichiometric displacement model and Langmuir and Freundlich adsorption. J. Chem. Educ. 79(3), 385 (2002). https://doi.org/10.1021/ed079p385
Osberghaus, A., Hepbildikler, S., Nath, S., Haindl, M., von Lieres, E., Hubbuch, J.: Determination of parameters for the steric mass action model-a comparison between two approaches. J. Chromatogr. A 1233, 54–65 (2012). https://doi.org/10.1016/j.chroma.2012.02.004
Chen, W.D., Hu, H.H., Wang, Y.D.: Analysis of steric mass-action model for protein adsorption equilibrium onto porous anion-exchange adsorbent. Chem. Eng. Sci. 61(21), 7068–7076 (2006). https://doi.org/10.1016/j.ces.2006.07.036
Gomes, P.F., Loureiro, J., Rodrigues, A.: Adsorption of human serum albumin (hsa) on a mixed-mode adsorbent: equilibrium and kinetics. Adsorption 23, 491–505 (2017). https://doi.org/10.1007/s10450-017-9861-x
Nogueira, K.A.B.: Study of biomolecules adsorption (serum albumin bovine and lysozyme) in mesoporous materials. Master’s thesis, Department of Chemical Engineering - UFC, http://www.repositorio.ufc.br/handle/riufc/23216 (2016)
Torres, M.A., Beppu, M.M., Santana, C.C.: Characterization of chemically modified chitosan microspheres as adsorbents using standard Proteins (bovine serum albumin and lysozyme). Braz. J. Chem. Eng. 24, 325–336 (2007)
Tong, X.D., Dong, X.Y., Sun, Y.: Lysozyme adsorption and purification by expanded bed chromatography with a small-sized dense adsorbent. Biochem. Eng. J. 12(2), 117–124 (2002). https://doi.org/10.1016/S1369-703X(02)00063-3
Salis, A., Ninham, B.: Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 43, 7358–7377 (2014). https://doi.org/10.1039/c4cs00144c
Tavares, F.W., Bratko, D., Blanch, H.W., Prausnitz, J.M.: Ion-specific effects in the colloid-colloid or protein-protein potential of mean force: Role of salt-macroion van der Waals interactions. J. Phys. Chem. B 108(26), 9228–9235 (2004). https://doi.org/10.1021/jp037809t
Tavares, F.W., Bratko, D., Prausnitz, J.M.: The role of salt-macroion van der Waals interactions in the colloid–colloid potential of mean force. Current Opin. Colloid Interface Sci. 9(1), 81–86 (2004). https://doi.org/10.1016/j.cocis.2004.05.008
Ståhlberg, J., Joensson, B., Horvath, C.: Theory for electrostatic interaction chromatography of proteins. Anal. Chem. 63(17), 1867–1874 (1991). https://doi.org/10.1021/ac00017a036
Parsegian, V., Gingell, D.: On the electrostatic interaction across a salt solution between two bodies bearing unequal charges. Biophys. J . 12(9), 1192–1204 (1972). https://doi.org/10.1016/S0006-3495(72)86155-1
Guélat, B., Ströhlein, G., Lattuada, M., Morbidelli, M.: Electrostatic model for protein adsorption in ion-exchange chromatography and application to monoclonal antibodies, lysozyme and chymotrypsinogen a. J. Chromatogr. A 1217(35), 5610–5621 (2010). https://doi.org/10.1016/j.chroma.2010.06.064
Guélat, B., Ströhlein, G., Lattuada, M., Delegrange, L., Valax, P., Morbidelli, M.: Simulation model for overloaded monoclonal antibody variants separations in ion-exchange chromatography. J. Chromatogr. A 1253, 32–43 (2012). https://doi.org/10.1016/j.chroma.2012.06.081
Moreira, L.A., Boström, M., Ninham, B.W., Biscaia, E.C., Tavares, F.W.: Effect of the ion–protein dispersion interactions on the protein–surface and protein–protein interactions. J. Braz. Chem. Soc. 18(1), 223–230 (2007). https://doi.org/10.1590/s0103-50532007000100026
Lima, E.R.A., Biscaia, E.C., Boström, M., Tavares, F.W., Prausnitz, J.M.: Osmotic second virial coefficients and phase diagrams for aqueous proteins from a much-improved Poisson–Boltzmann equation. J. Phys. Chem. C 111(43), 16055–16059 (2007). https://doi.org/10.1021/jp074807q
Alijó, P., Tavares, F., Biscaia, E., Jr.: Double layer interaction between charged parallel plates using a modified Poisson–Boltzmann equation to include size effects and ion specificity. Colloids Surf. A 412, 29–35 (2012). https://doi.org/10.1016/j.colsurfa.2012.07.008
Lima, E.R.A., Tavares, F.W., Biscaia, E.C., Jr.: Finite volume solution of the modified Poisson–Boltzmann equation for two colloidal particles. Phys. Chem. Chem. Phys. 9, 3174–3180 (2007). https://doi.org/10.1039/B701170A
Carnie, S.L., Chan, D.Y., Stankovich, J.: Computation of forces between spherical colloidal particles: Nonlinear Poisson–Boltzmann theory. J. Colloid Interface Sci. 165(1), 116–128 (1994). https://doi.org/10.1006/jcis.1994.1212
Gama, M., Simões Santos, M., Lima, E., Tavares, F., Barreto, A., Jr.: A modified Poisson–Boltzmann equation applied to protein adsorption. J. Chromatogr. A 1531, 74–82 (2018). https://doi.org/10.1016/j.chroma.2017.11.022
Vilarrasa-García, E., Cecilia, J., Santos, S., Cavalcante, C., Jiménez-Jiménez, J., Azevedo, D., Rodríguez-Castellón, E.: Co2 adsorption on aptes functionalized mesocellular foams obtained from mesoporous silicas. Microporous Mesoporous Mater. 187, 125–134 (2014). https://doi.org/10.1016/j.micromeso.2013.12.023
Sadler, P.J., Tucker, A.: pH-induced structural transitions of bovine serum albumin. Eur. J. Biochem. 212(3), 811–817 (1993). https://doi.org/10.1111/j.1432-1033.1993.tb17722.x
Lu, J.R., Su, T.J., Howlin, B.J.: The effect of solution pH on the structural conformation of lysozyme layers adsorbed on the surface of water. J. Phys. Chem. B 103(28), 5903–5909 (1999). https://doi.org/10.1021/jp990129z
Balme, S., Guegan, R., Janot, J., Jaber, M., Lepoitevin, M., Djardin, P., Bourrat, X., Motelica-Heino, M.: Structure, orientation and stability of lysozyme confined in layered materials. Soft Matter 9, 3188–3196 (2013). https://doi.org/10.1039/C3SM27880H
Zhao, D., Huo, Q., Feng, J., Chmelka, B.F., Stucky, G.D.: Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 120(24), 6024–6036 (1998). https://doi.org/10.1021/ja974025i
Ravikovitch, P.I., Neimark, A.V.: Characterization of micro- and mesoporosity in sba-15 materials from adsorption data by the nldft method. J. Phys. Chem. B 105(29), 6817–6823 (2001). https://doi.org/10.1021/jp010621u
Travalloni, L., Castier, M., Tavares, F.W., Sandler, S.I.: Thermodynamic modeling of confined fluids using an extension of the generalized van der Waals theory. Chem. Eng. Sci. 65(10), 3088–3099 (2010). https://doi.org/10.1016/j.ces.2010.01.032
Travalloni, L., Castier, M., Tavares, F.W.: Phase equilibrium of fluids confined in porous media from an extended Peng–Robinson equation of state. Fluid Phase Equilib. 362, 335–341 (2014). https://doi.org/10.1016/j.fluid.2013.10.049
Barbosa, G.D., DLima, M.L., Daghash, S.M., Castier, M., Tavares, F.W., Travalloni, L.: Cubic equations of state extended to confined fluids: New mixing rules and extension to spherical pores. Chem. Eng. Sci. 184, 52–61 (2018). https://doi.org/10.1016/j.ces.2018.03.047
Parsegian, V.: Van der waals forces: a handbook for biologists, chemists, engineers, and physicists. Van Der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists pp. 1–380 (2005). https://doi.org/10.1017/CBO9780511614606
Moon, P.H., Spencer, D.E.: Field Theory Handbook: Including Coordinate Systems, Differential Equations, and Their Solutions. Springer, Berlin (1961)
Stankovich, J., Carnie, S.L.: Electrical double layer interaction between dissimilar spherical colloidal particles and between a sphere and a plate: Nonlinear Poisson–Boltzmann theory. Langmuir 12(6), 1453–1461 (1996). https://doi.org/10.1021/la950384k
Ehrl, L., Jia, Z., Wu, H., Lattuada, M., Soos, M., Morbidelli, M.: Role of counterion association in colloidal stability. Langmuir 25(5), 2696–2702 (2009). https://doi.org/10.1021/la803445y
Lee, J.Y., Han, I.: A semi-empirical equation for activity coefficients of ions with one parameter. Bull. Korean Chem. Soc. 34(12), 3709–3714 (2013). https://doi.org/10.5012/bkcs.2013.34.12.3709
Pabst, T.M., Antos, D., Carta, G., Ramasubramanyan, N., Hunter, A.K.: Protein separations with induced pH gradients using cation-exchange chromatographic columns containing weak acid groups. J. Chromatogr. A 1181(1), 83–94 (2008). https://doi.org/10.1016/j.chroma.2007.12.054
Bonincontro, A., De Francesco, A., Onori, G.: Influence of pH on lysozyme conformation revealed by dielectric spectroscopy. Colloids Surf. B 12(1), 1–5 (1998). https://doi.org/10.1016/S0927-7765(98)00048-4
Hagemann, A., Giussi, J.M., Longo, G.S.: Use of pH gradients in responsive polymer hydrogels for the separation and localization of proteins from binary mixtures. Macromolecules 51(20), 8205–8216 (2018). https://doi.org/10.1021/acs.macromol.8b01876
Ide, M., El-Roz, M., De Canck, E., Vicente, A., Planckaert, T., Bogaerts, T., Van Driessche, I., Lynen, F., Van Speybroeck, V., Thybault-Starzyk, F., Van Der Voort, P.: Quantification of silanol sites for the most common mesoporous ordered silicas and organosilicas: total versus accessible silanols. Phys. Chem. Chem. Phys. 15, 642–650 (2013). https://doi.org/10.1039/C2CP42811C
Onizhuk, M., Panteleimonov, A., Kholin, Y., Ivanov, V.: Dissociation constants of silanol groups of silic acids: Quantum chemical estimations. J. Struct. Chem. 59, 261–271 (2018). https://doi.org/10.1134/S0022476618020026
Leite, F.L., Bueno, C.C., Da Róz, A.L., Ziemath, E.C., Oliveira, O.N.: Theoretical models for surface forces and adhesion and their measurement using atomic force microscopy. Int. J. Mol. Sci. 13(10), 12773–12856 (2012). https://doi.org/10.3390/ijms131012773
Leung, K., Nielsen, I.M.B., Criscenti, L.J.: Elucidating the bimodal acid–base behavior of the water–silica interface from first principles. J. Am. Chem. Soc. 131(51), 18358–18365 (2009). https://doi.org/10.1021/ja906190t
Kravchenko, A., Demianenko, E., Tsendra, O., Lobanov, V., Grebenyuk, A., Terets, M.: Simulation of the interaction between silica surface and acid or alkaline aqueous media. Collection 7(22), 36–41 (2015). ((In Ukrainian))
Yang, J., Su, H., Lian, C., Shang, Y., Liu, H., Wu, J.: Understanding surface charge regulation in silica nanopores. Phys. Chem. Chem. Phys. 22, 15373–15380 (2020). https://doi.org/10.1039/D0CP02152K
Salis, A., Bhattacharyya, M.S., Monduzzi, M.: Specific ion effects on adsorption of lysozyme on functionalized sba-15 mesoporous silica. J. Phys. Chem. B 114(23), 7996–8001 (2010). https://doi.org/10.1021/jp102427h
Franco, L.F.M., Pessôa Filho, P.D.A.: On the solubility of proteins as a function of pH: Mathematical development and application. Fluid Phase Equilibria 306(2), 242–250 (2011). https://doi.org/10.1016/j.fluid.2011.04.015
Bhattacharyya, M.S., Hiwale, P., Piras, M., Medda, L., Steri, D., Piludu, M., Salis, A., Monduzzi, M.: Lysozyme adsorption and release from ordered mesoporous materials. J. Phys. Chem. C 114(47), 19928–19934 (2010). https://doi.org/10.1021/jp1078218
Li, H., Robertson, A.D., Jensen, J.H.: Very fast empirical prediction and rationalization of protein pka values. Proteins 61(4), 704–721 (2005). https://doi.org/10.1002/prot.20660
Bas, D.C., Rogers, D.M., Jensen, J.H.: Very fast prediction and rationalization of pka values for protein-ligand complexes. Proteins 73(3), 765–783 (2008). https://doi.org/10.1002/prot.22102
Webb, H., Tynan-Connolly, B.M., Lee, G.M., Farrell, D., O’Meara, F., Søndergaard, C.R., Teilum, K., Hewage, C., McIntosh, L.P., Nielsen, J.E.: Remeasuring Hewl pka values by NMR spectroscopy: Methods, analysis, accuracy, and implications for theoretical pka calculations. Proteins 79(3), 685–702 (2011). https://doi.org/10.1002/prot.22886
Bonincontro, A., De Francesco, A., Onori, G.: Temperature-induced conformational changes of native lysozyme in aqueous solution studied by dielectric spectroscopy. Chem. Phys. Lett. 301(1), 189–192 (1999). https://doi.org/10.1016/S0009-2614(98)01460-2
Kubiak, K., Mulheran, P.A.: Molecular dynamics simulations of hen egg white lysozyme adsorption at a charged solid surface. J. Phys. Chem. B 113(36), 12189–12200 (2009). https://doi.org/10.1021/jp901521x
Salvalaglio, M., Paloni, M., Guelat, B., Morbidelli, M., Cavallotti, C.: A two level hierarchical model of protein retention in ion exchange chromatography. J. Chromatogr. A 1411, 50–62 (2015). https://doi.org/10.1016/j.chroma.2015.07.101
Zhang, J., Han, Y.T., Bu, X.L., Yue, X.F.: Study on the adsorption property of lysozyme on weak cation exchanger based on monodisperse poly(glycidymethacrylate-co-ethylenedimethacrylate) beads. J. Chromatogr. Sci. 51(2), 122–127 (2012). https://doi.org/10.1093/chromsci/bms115
Acknowledgements
The authors thank the financial support from the Brazilian National Agency of Petroleum, Natural Gas and Biofuels (ANP, Brazil) and PETROBRAS through the Clause of Investments in Research, Development, and Innovation. This study was financed by the National Council for Technological and Scientific Development (CNPq, Brazil) and the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). The authors thank all the comments and suggestions from the reviewers that much improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gama, M.d.S., Barreto, A.G. & Tavares, F.W. The binding interaction of protein on a charged surface using Poisson–Boltzmann equation: lysozyme adsorption onto SBA-15. Adsorption 27, 1137–1148 (2021). https://doi.org/10.1007/s10450-021-00344-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-021-00344-6