Abstract
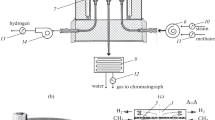
Steam reforming of methane and its mixtures containing 5 and 10% propane has been studied in a membrane reactor with an industrial nickel catalyst NIAP-03-01 and a membrane in the form of 30-μm foil made of a Pd–Ru alloy. At T = 823 K and a feed space velocity of 1800 h−1, the almost complete methane conversion is achieved, the selectivity for CO2 is more than 50%, and about 80% H2 is recovered from the reaction mixture. High conversion of CH4 in the membrane reactor under mild conditions allows the steam reforming of its mixtures with C2+ alkanes to be conducted in a single process, as shown by the example of model mixtures containing C3H8. Under selected conditions (T = 773 or 823 K, a feed space velocity of 1800 or 3600 h−1, a steam/methane ratio of 3 or 5, atmospheric pressure), almost complete C3H8 conversion is observed. The main “undesirable” reaction is methanation, leading to a decrease in the CH4 conversion. In the system under study, CH4 is formed with an increase in the feed space velocity. Methanation occurs as a result of C3H8 hydrocracking at a steam/feedstock ratio = 3 or the hydrogenation of CO2 as this ratio is increased to 5. The optimal conditions for steam reforming of methane mixtures containing up to 10% C3H8 are T = 823 K, steam/feedstock ratio = 5, and the feed space velocity of 1800 h−1. Under these conditions involving evacuation of the permeate, the feedstock conversion is complete, the selectivity for CO2 is 50%, and more than 70% H2 is recovered from the reaction mixture.



Similar content being viewed by others
REFERENCES
S. J. Peighambardoust, S. Rowshanzamir, and M. Amjjadi, J. Hydrogen Energy 35, 9349 (2010).
S. A. Skrylev, Energ. Strateg., No. 2, 15 (2012).
I. V. Kozodoev, N. A. Kuzin, Yu. I. Amosov, et al., Ind. Ecol. North. 11, 40 (2011).
G. S. Fal’kovich, N. N. Rostanin, L. M. Vilenskii, et al., Katal. Prom-sti, No. 3, 10 (2003).
V. S. Arutyunov and O. V. Krylov, Oxidative Conversion of Methane (Nauka, Moscow, 1998) [in Russian].
K. Aasberg-Petersen, I. Dybkjaer, C. V. Ovesen, et al., J. Nat. Gas Sci. Eng. 3, 423 (2011).
S. Yoon and J. Bae, Catal. Today 156, 49 (2010).
X. Wang, X. Zou, X. Wang, et al., Int. J. Hydrogen Energy 36, 4908 (2011).
B. T. Schadel, M. Duisberg, and O. Deutschmann, Catal. Today 142, 42 (2009).
Y. Chen, Y. Wang, H. Xu, and G. Xiong, Appl. Catal., B 80, 283 (2008).
M. Saric, Y. C. Delft, R. Sumbharaju, et al., Catal. Today 193, 74 (2012).
J. Y. Matsumura and J. Tong, Top. Catal. 51, 283 (2008).
G. Burkhanov, N. Gorina, N. Kolchugina, and N. Roshan, Platinum Met. Rev. 55, 3 (2011).
L. Didenko, V. Savchenko, L. Sementsova, and L. Bikov, Int. J. Hydrogen Energy 41, 307 (2016).
V. M. Ievlev, K. A. Solntsev, A. I. Dontsov, et al., Tech. Phys. 61, 467 (2016).
V. M. Ievlev, A. A. Maksimenko, A. I. Sitnikov, et al., Materialovedenie, No. 2, 37 (2016).
V. M. Ievlev, G. S. Burkhanov, A. A. Maksimenko, et al., Kondens. Sredy Mezhfaz. Granitsy 15, 121 (2013).
Y. Shirasaki, T. Tsuneki, Y. Ota, et al., Int. J. Hydrogen Energy 34, 4482 (2009).
L. P. Didenko, L. A. Sementsova, P. E. Chizhov, et al., Russ. Chem. Bull. 65, 1997 (2016).
S. Campanari, E. Macchi, and G. Manzolini, Int. J. Hydrogen Energy 33, 1361 (2008).
P. Ciavarella, D. Casanave, H. Moueddeb, et al., Catal. Today 67, 177 (2001).
L. van Dyk, S. Miachon, L. Lorenzen, et al., Catal. Today 82, 167 (2003).
S. Miachon and J.-A. Dalmon, Top. Catal. 29, 59 (2004).
L. P. Didenko, V. I. Savchenko, L. A. Sementsova, and P. E. Chizhov, Pet. Chem. 56, 459 (2016).
M. V. Zyryanova, P. V. Snytnikov, A. B. Shigarov, et al., Fuel 135, 76 (2014).
M. M. Zyryanova, S. D. Badmaev, V. D. Belyaev, Yu. I. Amosov, P. V. Snytnikov, V. A. Kirillov, V. A. Sobyanin, Catal. Ind. 5, 312 (2013).
B. T. Schadel, M. Duisberg, and O. Deutschmann, Catal. Today 142, 42 (2009).
M. A. Rarib, J. R. Grace, C. J. Lim, and S. S. E. H. Elnashaie, Int. J. Hydrogen Energy 35, 6276 (2010).
ACKNOWLEDGMENTS
This work was carried out under the 2013–2020 Program of Basic Research of State Academies of Sciences, theme code 0089-2014-0032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Didenko, L.P., Sementsova, L.A., Chizhov, P.E. et al. Steam Reforming of Methane and Its Mixtures with Propane in a Membrane Reactor with Industrial Nickel Catalyst and Palladium–Ruthenium Foil. Pet. Chem. 59, 394–404 (2019). https://doi.org/10.1134/S0965544119040054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544119040054