Abstract
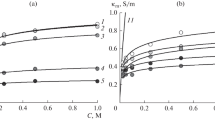
The effect of pH and concentration of the NaCl solution on the exchange capacity and transport properties of the MA-40 and MA-41 anion-exchange heterogeneous membranes with different nature of fixed groups has been studied: MA-40 has weakly basic groups, and MA-41 has strongly basic groups with a small proportion of weakly basic ones. The exchange capacity, thickness, water content, and electrical conductivity of the membranes equilibrated with NaCl solutions of various concentrations and pH were measured; for the same solutions, the diffusion permeability coefficients were found. The capacity was measured in the pH range from 1.5 to 12; the remaining properties, in the pH range from 3 to 9. Using the values of electrical conductivity and diffusion permeability, the ion transport numbers in the membranes were calculated. It was shown that at the external solution pH 9 the thickness of the membranes and their electric conductivity are minimal, and the transport numbers of co-ions are maximum. This is explained by the fact that in basic solutions weakly basic functional groups are largely deprotonated, and the effective capacity of the membrane is significantly reduced. The maximum effective capacity is achieved at pH \( \leqslant \) 3; in this case, transport numbers of co-ions in the MA-40 membrane are 5-fold, and in the MA-41 membrane, two-fold lower than corresponding values at pH 6 and 9. The changes in the transport properties of the membranes with increasing pH are due to a decrease in the degree of protonation of weakly basic functional groups, these changes are more pronounced for the MA-40 membrane than for MA-41.






Similar content being viewed by others
REFERENCES
N. P. Gnusin, V. I. Zabolotskii, V. V. Nikonenko, and A. I. Meshechkov, Zh. Fiz. Khim. 54, 1518 (1980).
N. P. Gnusin, N. P. Berezina, N. A. Kononenko, and O. A. Dyomina, J. Membr. Sci. 243, 301 (2004).
N. P. Berezina, N. A. Kononenko, O. A. Dyomina, and N. P. Gnusin, Adv. Colloid Interface Sci. 139, 3 (2008).
N. D. Pismenskaya, E. I. Belova, V. V. Nikonenko, and C. Larchet, Russ. J. Electrochem. 44, 1285 (2008).
V. K. Shahi, A. P. Murugesh, B. S. Makwana, et al., Indian J. Chem. A 39, 1264 (2000).
X. Tongwen and Y. Weihua, J. Membr. Sci. 190, 159 (2001).
L. X. Tuan, J. Colloid Interface Sci. 325, 215 (2008).
Y. Sedkaoui, A. Szymczyk, H. Lounici, and O. Arous, J. Membr. Sci. 507, 34 (2016).
V. I. Zabolotsky and V. V. Nikonenko, J. Membr. Sci. 79, 181 (1993).
F. Helfferich, Ionenaustauscher Bd. 1: Grundlagen Struktur-Herstellung-Theorie (Chemie, Weinheim, 1959).
N. D. Pis’menskaya, Russ. J. Electrochem. 32, 252 (1996).
G. Yu. Lopatkova, E. I. Volodina, N. D. Pis’menskaya, et al., Russ. J. Electrochem. 42, 847 (2006).
V. I. Zabolotskii, S. V. Utin, N. V. Shel’deshov, K. A. Lebedev, P. A. Vasilenko, Russ. J. Electrochem. 47, 321 (2011).
N. Kononenko, V. Nikonenko, D. Grande, et al., Adv. Colloid Interface Sci. 246, 196 (2017).
T. K. Brutskus, E. V. Zambrovskaya, I. V. Sambrovskii, and A. B. Pashkov, Ion-Exchange Resins: A Catalogue (NIITEKhim, Cherkassy, 1975) [in Russian].
J. Balster, I. Punt, D. F. Stamatialis, et al., J. Membr. Sci. 303, 213 (2007).
V. Zabolotskii, N. Sheldeshov, and S. Melnikov, Desalination 342, 183 (2014).
S. A. Loza, V. I. Zabolotsky, N. V. Loza, and M. A. Fomenko, Pet. Chem. 56, 1027 (2016).
S. Mikhaylin, V. Nikonenko, G. Pourcelly, and L. Bazinet, Green Chem. 18, 307 (2016).
V. Zabolotsky, S. Utin, A. Bespalov, and V. Strelkov, J. Membr. Sci. 494, 188 (2015).
E. M. Akberova, Candidate’s Dissertation in Chemistry (Voronezh, 2015) [in Russian].
O. A. Demina, N. P. Berezina, T. Sata, and A. V. Demin, Russ. J. Electrochem 38, 896 (2002).
V. I. Vasil’eva, N. D. Pismenskaya, E. M. Akberova, and K. A. Nebavskaya, Russ. J. Phys. Chem. 88, 1293 (2014).
V. D. Grebenyuk and A. A. Mazo, Desalination of Water with Ion-Exchange Resins (Khimiya, Moscow, 1980) [in Russian].
E. E. Nevakshenova, Candidate’s Dissertation in Chemistry (Krasnodar, 2013) [in Russian].
Ion-Exchange Resin Membranes, Granulates, and Powders: A Catalogue (NIITEKhim, Moscow, 1977) [in Russian]..
E. M. Akberova and M. D. Malykhin, Sorb. Khromatogr. Protsess. 14, 232 (2014).
G. Merle, M. Wessling, and K. Nijmeijer, J. Membr. Sci. 377, 1 (2011).
N. N. Belaid, L. Dammak, B. Ngom, et al., Eur. Polym. J. 34, 564 (1998).
L. V. Karpenko, O. A. Demina, G. A. Dvorkina, et al., Russ. J. Electrochem. 37, 287 (2001).
V. V. Nikonenko, RU Patent No. 2010121195 (2010).
N. P. Gnusin, N. P. Berezina, A. A. Shudrenko, and O. P. Ivina, Zh. Fiz. Khim. 68, 565 (1994).
V. I. Zabolotskii and V. V. Nikonenko, Ion Transport in Membranes (Nauka, Moscow, 1996) [in Russian].
C. Larchet, L. Dammak, B. Auclair, et al., New J. Chem. 28, 1260 (2004).
S. A. Lawrence, Amines: Synthesis, Properties and Applications (Cambridge University Press, Cambridge, 2004).
L. Franck-Lacaze, P. Sistat, P. Huguet, and F. Lapicque, J. Membr. Sci. 340, 257 (2009).
V. Sarapulova, E. Nevakshenova, N. Pismenskaya, et al., J. Membr. Sci. 479, 28 (2015).
B. B. Damaskin, O. A. Petrii, and G. A. Tsirlina, Textbook of Electrochemistry (Khimiya, Moscow, 2001) [in Russian].
ACKNOWLEDGMENTS
The authors thank Professor N.V. Shel’deshov for valuable advice and useful discussions. The work was supported by the Russian Science Foundation, project no. 17-19-014-86.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhaev
Rights and permissions
About this article
Cite this article
Zyryanova, S.V., Pismenskaya, N.D. & Nikonenko, V.V. The Effect of Concentration and pH of NaCl Solution on the Transport Properties of Anion Exchange Membranes with Different Fixed Groups. Pet. Chem. 58, 965–974 (2018). https://doi.org/10.1134/S0965544118110087
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544118110087