Abstract
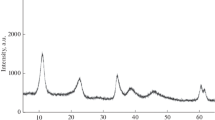
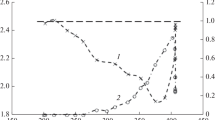
Partial oxidation of methane and dry methane reforming to synthesis gas in the presence of catalysts based on hydrotalcite-like hydroxo salts [AlMg2Ni x Co y (OH)6.08][(NO3) n H2O], where x = 0, 0.02, 0.04 and y = 0, 0.02, 0.04 with a total Ni and/or Co content of no more than 2 wt % have been first studied. It has been shown that the Ni-containing catalysts provide a synthesis gas yield of 90 and 97% in the case of partial oxidation and dry reforming of methane, respectively; in the presence of these catalysts, a trace amount of carbon nanotubes is formed; the catalyst sample containing both nickel and cobalt does not lead to the formation of any carbon nanotubes during dry reforming of methane.
Similar content being viewed by others
References
A. Holmen, Catal. Today 142, 2 (2009).
B. C. Enger, R. Lodeng, and A. Holmen, Appl. Catal., A 346, 1 (2008).
S. Zeng, X. Zhang, X. Fu, et al., Appl. Catal., B 136/137, 308 (2013).
A. G. Dedov, A. S. Loktev, D. A. Komissarenko, et al., Fuel Process. Technol. 148, 128 (2016).
A. G. Dedov, A. S. Loktev, V. K. Ivanov, et al., Dokl. Phys. Chem. 461, 73 (2015).
F. Basile, L. Basini, M. D’Amore, et al., J. Catal. 173, 247 (1998).
K. M. Lee and W. Y. Lee, Catal. Lett. 83, 65 (2002).
K. Takehira, T. Shishido, P. Wan, et al., J. Catal. 221, 43 (2004).
T. P. Maniecki, K. Bawolak-Olczak, P. Mierczyn’ski, et al., Chem. Eng. J. 154, 142 (2009).
J. R. Rostrup-Nielsen and J. H. Bak Hansen, J. Catal. 144, 38 (1993).
J. Guo, H. Lou, H. Zhao, et al., Appl. Catal., A 273, 75 (2004).
A. Bhattacharyya, V. W. Chang, and D. J. Schumacher, Appl. Clay Sci. 13, 317 (1998).
T. Shishido, M. Sukenobu, H. Morioka, et al., Catal. Lett. 73, 21 (2001).
A. I. Tsyganok, T. Tsunoda, S. Hamakawa, et al., J. Catal. 213, 191 (2003).
Z. Hou and T. Yashima, Appl. Catal., A 261, 205 (2004).
O. W. Perez-Lopez, A. Senger, N. R. Marcilio, and M. A. Lansarin, Appl. Catal., A 303, 234 (2006).
A. Serrano-Lotina, L. Rodriguez, G. Muñoz, and L. Daza, J. Power Sources 196, 4404 (2011).
A. Serrano-Lotina, A. J. Martin, M. A. Folgado, and L. Daza, Int. J. Hydrogen Energy 37, 12342 (2012).
A. Serrano-Lotina and L. Daza, J. Power Sources 238, 81 (2013).
A. Serrano-Lotina and L. Daza, Appl. Catal., A 474, 107 (2014).
G. de Souza, C. Ruoso, N. R. Marcilio, and O. W. Perez-Lopez, Chem. Eng. Commun. 203, 783 (2016).
C. Tanios, S. Bsaibes, C. Gennequin, et al., Int. J. Hydrogen Energy 42, 12818 (2017).
O. N. Krasnobaeva, I. P. Belomestnykh, G. V. Isagulyants, et al., Russ. J. Inorg. Chem. 52, 141 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.G. Dedov, A.S. Loktev, V.P. Danilov, O.N. Krasnobaeva, T.A. Nosova, I.E. Mukhin, S.I. Tyumenova, A.E. Baranchikov, V.K. Ivanov, M.A. Bykov, I.I. Moiseev, 2018, published in Neftekhimiya, 2018, Vol. 58, No. 3.
Rights and permissions
About this article
Cite this article
Dedov, A.G., Loktev, A.S., Danilov, V.P. et al. Catalytic Materials Based on Hydrotalcite-Like Aluminum, Magnesium, Nickel, and Cobalt Hydroxides for Partial Oxidation and Dry Reforming of Methane to Synthesis Gas. Pet. Chem. 58, 418–426 (2018). https://doi.org/10.1134/S0965544118050055
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544118050055