Abstract
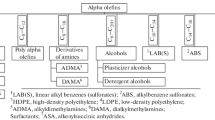
Main methods for the polymerization of higher α-olefins (1-hexene, 1-octene, and 1-decene) with the formation of high molecular weight products, key properties of poly-α-olefins from the viewpoint of their use as drag reducing agents in the transportation of petroleum and petroleum products, as well as the challenges and prospects of the production of these important functional materials of the petroleum industry have been considered.
Similar content being viewed by others
References
B. A. Toms, in Proceedings of the 1st International Congress on Rheology (1949), No. 2, p. 135.
Y. Wang, B. Yu, J. L. Zakin, and H. Shi, Adv. Mech. Eng., ID 478749 (2011).
P. S. Virk, AIChE J. 21, 625 (1975).
C. M. White and M. G. Mungal, Ann. Rev. Fluid Mech. 40, 235 (2008).
W. J. Brostow, Ind. Eng. Chem. 14, 409 (2008).
G. V. Nesyn, A. G. Postoev, V. L. Kuznetsov, et al., RU Patent No. 2 075 485 (1997).
E. J. Arlman and P. J. Cossee, Catalysis 3, 99 (1964).
T. Asakura, M. Demura, and Y. Nishiyama, Macromolecules 24, 2334 (1991).
U. Subramanyam, P. R. Rajamohanan, and S. Sivaram, Polymer 45, 4063 (2004).
N. Kawahara, J. Saito, S. Matsuo, et al., Polymer 48, 425 (2007).
J. D. Azoulay, G. C. Bazan, and G. B. Galland, Macromolecules 43, 2794 (2010).
X. Zhao, G. Odian, and A. Rossi, J. Polym. Sci., Part A: Polym. Chem. 38, 3802 (2000).
K. A. Novstrup, N. E. Travia, G. A. Medvedev, et al., J. Am. Chem. Soc. 132, 558 (2010).
S. Budagumpi, K.-H. Kim, and I. Kim, Coord. Chem. Rev. 255, 2785 (2011).
C. Redshaw and Y. Tang, Chem. Soc. Rev., No. 41, 4484 (2012).
H. Makio, H. Terao, A. Iwashita, and T. Fujita, Chem. Rev. 111, 2363 (2011).
Y.-Y. G. Luk, D. A. Foucher, and R. A. Gossage, C. R. Chim. 16, 573 (2013).
M. Hofman and K. Nomura, J. Mol. Catal. A: Chem. 319, 85 (2010).
S. Segal, I. Goldberg, and M. Kol, Organometallics 24, 200 (2005).
I. Haas, T. Dietel, K. Press, et al., Chem. Eur. J. 19, 14254 (2013).
L. Resconi, E. Ciaccia, F. Morhard, and G. Pellegatti, US Patent No. 2008281060 (2008).
E. J. Badin, J. Am. Chem. Soc. 80, 6549 (1958).
D. K. Dandge, J. P. Heller, C. Lien, and K. V. Wilson, J. Appl. Polym. Sci. 32, 5373 (1986).
M. P. Mack, US Patent No. 4 493903 (1985).
M. P. Mack, US Patent No. 4 289 679 (1981).
M. P. Mack, L. B. Decker, and A. L. Wallace, US Patent No. 4 358 572 (1982).
M. P. Mack, US Patent No. 4 415 714 (1983).
C. T. Berge and S. L. Baxter, EPA Patent No. 0133214 (1984).
M. P. Mack, US Patent No. 4 433 123 (1984).
G. V. Nesyn, A. M. Shiryaev, M. I. Valiev, et al., RU Patent No. 2481357 (2013).
R. M. Kowalik, I. Duvdevani, K. Kitano, and D. N. Schulz, US Patent No. 4 625 745 (1986).
K. W. Smith, L. V. Haynes, and D. F. Massouda, US Patent No. 5504132 (1996).
R. L. Johnson and S. L. Milligan, US Patent No. 2003 069 330 (2003).
S. N. Milligan and K. W. Smith, US Patent No. 6 576 732 (2003).
K. W. Smith, L. V. Haynes, and D. F. Massouda, WO Patent No. 9500563 (1995).
G. V. Nesyn, RU Patent No. 2 238 282 (2004).
N. S. Kommareddi, G. G. Ramsay, and J. F. Motler, US Patent No. 2004 167 297 (2004).
J. R. Harris, US Patent No. 2006 281 832 (2006).
V. A. Zakharov, S. A. Sergeev, L. G. Echevskaya, and V. N. Manzhai, RU Patent No. 2 230 074. (2004).
R. N. Haward, A. N. Roper, and K. L. Fletcher, Polymer 14, 365 (1973).
P. K. Saxena, Eur. Polym. J. 35, 1313 (1999).
I. V. Vasilenko and S. V. Kostjuk, Polym. Bull. 57, 129 (2006).
X. Jiang and Z. Fan, Chin. J. Polym. Sci. 22, 305 (2004).
X. Jiang, X. Tian, Z. Fan, et al., J. Mol. Catal. A: Chem. 275, 72 (2007).
L. Echevskaya, M. Matsko, M. Nikolaeva, et al., Macromol. React. Eng. 8, 666 (2014).
S. A. Sergeev, G. D. Bukatov, and V. A. Zakharov, RU Patent No. 2 191 196 (2002).
Z. Fan, L. Zhang, S. Xia, and Z. Fu, J. Mol. Catal. A: Chem. 351, 93 (2011).
H. Harjuhahto, E. Virtanen, A. K. Karbasi, et al., WO Patent No. 0234802 (2002).
R. L. Johnston, US Patent No. 4 756 326 (1988).
J. M. Pomeroy, US Patent No. 4 771 800 (1988).
S. L. Baxter and M. H. Lewis, US Patent No. 4 771799 (1988).
I. V. Prozorova, N. V. Yudina, Yu. V. Loskutova, et al., RU Patent No. 2 225 434 (2004).
D. P. O’Mara, A. F. Hadermann, and J. C. Trippe, US Patent No. 4 720 397 (1988).
K. M. Labude, K. W. Smith, and T. L. Burden, US Patent No. 6 399 676 (2002).
K. W. Smith, L. V. Haynes, and D. F. Massouda, US Patent No. 5 504 131 (1996).
R. L. Johnson and Y. N. Lee, US Patent No. 6 172 151 (2001).
K. W. Smith, S. Milligan, R. L. Johnston, and J. L. Krottinger, WO Patent No. 0 243 849 (2002).
G. B. Eaton and A. K. Ebert, US Patent No. 2002 198 116 (2002).
R. L. Johnson, S. L. Milligan, and K. W. Smith, US Patent No. 2002 065 352 (2002).
T. J. Martin and L. C. Chou, US Patent No. 2007066713 (2007).
T. J. Martin, US Patent No. 2007 205 392 (2007).
G. B. Eaton and A. K. Ebert, US Patent No. 2011105642 (2011).
B. A. Bucher and T. M. Weatherford, US Patent No. 2014039229 (2014).
G. L. Lobanova, V. V. Lopatin, G. V. Nesyn, et al., RU Patent No. 2 314 912 (2008).
K. B. Konovalov, N. M. Polyakova, and G. V. Nesyn, RU Patent No. 2 463 320 (2012).
B. Li, W. Xing, G. Dong, et al., Pet. Sci. 8, 99 (2011).
K. Fairchild, R. Tipton, J. F. Motier, and N. S. Kommareddi, US Patent No. 5 733 953 (1998).
N. S. Kommareddi and L. J. Rzeznik, US Patent No. 6 160 036 (2000).
E. Karhu, M. Karhu, L. Rockas, and H. Harjuhahto, US Patent No. 2002 173 569 (2002).
B. Liu, X. Bao, Y. Gao, et al., US Patent No. 2007004837 (2007).
J. F. Motier, L.-C. Chou, and C. L. Tong, US Patent No. 2004 254 266 (2004).
T. Mathew, K. D. Fairchild, and N. S. Kommareddi, US Patent No. 2007 066 712 (2007).
J. R. Harris and J. F. Motier, US Patent No. 2004112995 (2004).
T. Mathew and N. S. Kommareddi, US Patent No. 2008287568 (2008).
G. V. Nesyn, A. M. Shiryaev, M. I. Valiev, et al., US Patent No. 2014 228 529 (2014).
G. B. Eaton and M. J. Monahan, US Patent No. 5 869 570 (1999).
G. V. Nesyn, Y. V. Souleymanova, and V. S. Stankevich, in Proceedings of VII Annual European Rheological Conference (2011), p. 125.
G. V. Nesyn, V. S. Stankevich, Yu. V. Suleimanova, et al., RU Patent No. 2443720 (2012).
T. M. Zilberstein, M. V. Lipskikh, A. A. Nosikov, and G. V. Nesyn, US Patent No. 2012 302 715 (2012).
A. Forestiere, H. Olivier-Bourbigou, and L. Saussine, Oil Gas Sci. Technol. 64, 649 (2009).
K. P. Bryliakov and E. P. Talsi, Coord. Chem. Rev. 256, 2994 (2012).
J. T. Dixon, M. J. Green, F. M. Hess, and D. H. Morgan, J. Organomet. Chem. 689, 3641. (2004).
G. P. Belov, Pet. Chem. 52, 139 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.V. Ivchenko, I.E. Nifant’ev, A.V. Tavtorkin, 2016, published in Neftekhimiya, 2016, Vol. 56, No. 6, pp. 553–566.
Rights and permissions
About this article
Cite this article
Ivchenko, P.V., Nifant’ev, I.E. & Tavtorkin, A.V. Polyolefin drag reducing agents (Review). Pet. Chem. 56, 775–787 (2016). https://doi.org/10.1134/S096554411609005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S096554411609005X