Abstract
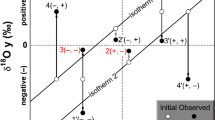
The behavior of four isotope systems (δ18О, δ13С, δ88Sr and 87Sr/86Sr) during submarine precipitation of inorganic carbonates is considered using the Lost City hydrothermal field as an example of “natural laboratory”. During the carbonate precipitation, the isotope composition, T, and pH of hydrothermal solution change due to mixing of the “end member” Lost City fluid with ocean water. The composition of DIC and carbonates (Cc, Arag) equilibrated with mixed hydrothermal solution was calculated in the isotopic coordinates 87Sr/86Sr–103(Sr/Ca), 87Sr/86Sr–δ18О, 87Sr/86Sr–δ13С, and 87Sr/86Sr–δ88Sr. The observed isotope composition of the Lost City field carbonates is compared with the calculated equilibrium lines. The disequilibrium values of δ18О, δ13С, and δ88Sr in the Cс(Arag) are result of rapid precipitation of carbonates from a hydrothermal fluid under T and pH gradient. The δ18О values of most chimney samples vary around the “DIC–water” equilibrium curve with a slight shift to the Сс(Arag)–water equilibrium. The values of δ13С of chimney carbonates fall between the calculated curves of δ13С (DIC) and equilibrium Сс and Arag. The kinetic isotopic shift Δ88Sr established in chimney carbonates is close to available experimental data on the synthesis of calcite and aragonite from aqueous solutions.









Similar content being viewed by others
Notes
DIC = [CO2]aq + [H2CO3]0 + [HCO3]– + [CO3]2–).
It is necessary to note that this shift does not exert significant influence on the results of mixing calculations, where 87Sr/86Sr was used as an indicator of LCF and SW mass fractions. The contribution of 88Sr/86Sr in the measurement error of 87Sr/86Sr is insignificant, since the scale of 87Sr/86Sr variations (in rel %) in the studied samples is over an order of magnitude higher than that of 88Sr/86Sr. For instance, at a maximum shift measured in this work (Δ88Sr(сarb-w) = –0.34‰), a possible shift of 87Sr/86Sr ratio caused by mass-dependent fractionation is as low as –0.0001 (according to Eqs. (19–21) from Young et al., 2002).
REFERENCES
AlKhatib, M. and Eisenhauer, A., Calcium and strontium isotope fractionation in aqueous solutions as a function of temperature and reaction rate; I. Calcite, Geochim. Cosmochim. Acta, 2017a, vol. 209, pp. 296–319.
AlKhatib, M. and Eisenhauer, A., Calcium and strontium isotope fractionation during precipitation from aqueous solutions as a function of temperature and reaction rate; II. Aragonite, Geochim. Cosmochim. Acta, 2017b, vol. 209, pp. 320–342.
Allen, D.E. and Seyfried, W.E., Serpentinization and heat generation: constraints from Lost City and Rainbow hydrothermal systems, Geochim. Cosmochim. Acta, 2004, vol. 68, no. 6, pp. 1347–1354.
Beck, W.C., Grossman, E.L., and Morse, J.W., Experimental studies of oxygen isotope fractionation in the carbonic acid system at 15°, 25°, and 40°C, Geochim. Cosmochim. Acta, 2005, vol. 69, no. 14, pp. 3493–3503.
Böhm, F., Eisenhauer, A., Tang, J., et al., Strontium isotope fractionation of planktic foraminifera and inorganic calcite, Geochim. Cosmochim. Acta, 2012, vol. 93, pp. 300–314.
Bonatti, E., Anomalous opening of the Equatorial Atlantic due to an equatorial mantle thermal minimum, Earth Planet. Sci. Lett., 1996, vol. I43, pp. 147–160.
Boschi, C., Dini, A., Fruh-Green, G.L., and Kelley, D.S., Isotopic and element exchange during serpentinization and metasomatism at the Atlantis Massif (MAR, 30° N): insights from B and Sr isotope data, Geochim. Cosmochim. Acta, 2008, vol. 72, pp. 1801–1823.
Cannat, M., Lagabrielle, Y., Bougault, H., et al., Ultramafic and gabbroic exposures at the Mid-Atlantic Ridge: geologic mapping in the 15° N region, Tectonophysics, 1997, vol. 279 P, pp. 193–213.
Chacko, T. and Deines, P., Theoretical calculation of oxygen isotope fractionation factors in carbonate systems, Geochim. Cosmochim. Acta, 2008, vol. 72, pp. 3642–3660.
Charlier, B.L.A., Nowell, G.M., Parkinson, I.J., et al., High temperature strontium stable isotope behaviour in the early solar system and planetary bodies, Earth Planet. Sci. Lett., 2012, vol. 329-330, pp. 31–40.
Coplen, T.B., Calibration of the calcite–water oxygen–isotope geothermometer at Devils Hole, Nevada, a natural laboratory, Geochim. Cosmochim. Acta, 2007, vol. 71, pp. 3948–3957.
Day, C.C. and Henderson, G.M., Oxygen isotopes in calcite grown under cave-analogue conditions, Geochim. Cosmochim. Acta, 2011, vol. 75, pp. 3956–3972.
Delacour, A., Fruh-Green, G.L., Frank, M., et al., Sr- and Nd-isotope geochemistry of the Atlantis Massif (30° N, MAR): implications for fluid fluxes and lithospheric heterogeneity, Chem. Geol., 2008, vol. 254, nos. 1–2, pp. 19–35.
Devriendt, L.S., Watkins, J.M., and McGregor, H.V., Oxygen isotope fractionation in the CaCO3–DIC–H2O system, Geochim. Cosmochim. Acta, 2017, vol. 214 P, pp. 115–142.
Dietzel, M., Jianwu, T., Leis, A., and Kohler, S.J., Oxygen isotopic fractionation during inorganic calcite precipitation? Effects of temperature, precipitation rate and pH, Chem. Geol., 2009, vol. 268, nos. 1–2, pp. 107–115.
Dubinina, E.O., Chernyshev, I.V., Bortnikov, N.S., et al., Isotopic–geochemical characteristics of the Lost City hydrothermal field, Geochem. Int., 2007, vol. 45, no. 11, pp. 1131–1143.
Dubinina, E.O., Bortnikov, N.S., and Silantyev, S.A., Fluid–rock interaction during seprentinization of oceanic ultramafic rocks hosting the Lost City hydrothermal field, 30° N, MAR, Petrology, 2015, vol. 23, no. 6, pp. 543–558.
Dubinina, E.O., Kossova, S.A., Miroshnikov, A.Yu., and Kokryatskaya, N.M., Isotope (δD, δ18O) systematics in waters of the Russian Arctic seas, Geochem. Int., 2017, vol. 55, no. 11, pp. 1022–1032.
Elderfield, H. and Schultz, A., Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean, Ann. Rev. Eart. Planet. Sci., 1996, vol. 24, pp. 191–224.
Fietzke, J. and Eisenhauer, A., Determination of temperature-dependent stable strontium isotope (88Sr/86Sr) fractionation via bracketing standard MC-ICP-MS, Geochem. Geophys. Geosyst., 2006, vol. 7, no. 8, p. Q08009. https://doi.org/10.1029/2006GC001243
Foustoukos, D.I., Savov, I.P., and Janecky, D.R., Chemical and isotopic constraints on water/rock interactions at the Lost City hydrothermal field, 30° N Mid-Atlantic Ridge, Geochim. Cosmochim. Acta, 2008, vol. 72, pp. 5457–5474.
Früh-Green, G.L., Kelley, D.S., Bernasconi, S.M., et al., 30 000 years of hydrothermal activity at the Lost City vent field, Science, 2003, vol. 301, pp. 495–498.
Früh-Green, G.L., Delacour, A., Boschi, C., et al., Building Lost City: serpentinization, mass transfer and life in a peridotite-hosted hydrothermal system, Geochim. Cosmochim. Acta, 2007, vol. 71, p. A298.
Gabitov, R.I. and Watson, E.B., Partitioning of strontium between calcite and fluid, Geochem., Geophys., Geosyst., 2006, vol. 7, no. 11, p. Q11004.
Gabitov, R.I., Watson, E.B., and Sadekov, A., Oxygen isotope fractionation between calcite and fluid as a function of growth rate and temperature: an in situ study, Chem. Geol., 2012, vol. 306–307, pp. 92–102.
Gaetani, G.A. and Cohen, A.L., Element partitioning during precipitation of aragonite from seawater: a framework for understanding paleoproxies, Geochim. Cosmochim. Acta, 2006, vol. 70, no. 18, pp. 4617–4634.
Karson, J.A., Früh-Green, G.L., Kelley, D.S., et al., Detachment shear zone of the Atlantis Massif core complex, Mid-Atlantic Ridge, 30° N, Geochem., Geophys., Geosyst., 2006, vol. 7, p. Q06016.
Kelley, D.S., Karson, J.A., Blackman, D.K., et al., An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N, Nature, 2001, vol. 412, no. 12, pp. 145–149.
Kelley, D.S., Karson, J.A., Früh-Green, G.L., et al., A serpentinite-hosted ecosystem: the Lost City hydrothermal field, Science, 2005, vol. 307, pp. 1428–1434.
Kim, S.-T. and O’Neil, J.R., Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates, Geochim. Cosmochim. Acta, 1997, vol. 61, no. 16, pp. 3461–3475.
Kim, S.T., O’Neil, J.R., Hillaire-Marcel, C., and Mucci, A., Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration, Geochim. Cosmochim. Acta, 2007, vol. 71, pp. 4704–4715.
Kinsman, D.J.J. and Holland, H.D., The coprecipitation of cations with CaCO3—IV. The coprecipitation of Sr+2 with aragonite between 16o and 96o, Geochim. Cosmochim. Acta, 1969, vol. 33, pp. 1–17.
Krabbenhöft, A., Eisenhauer, A., Bohm, F., et al., Constraining the marine strontium budget with natural strontium isotope fractionations (87Sr/86Sr*, δ 88/86Sr) of carbonates, hydrothermal solutions and river waters, Geochim. Cosmochim. Acta, 2010, vol. 74, no. 14, pp. 4097–4109.
Kramchaninov, A.Yu., Chernyshev, I.V., and Shatagin, K.N., Strontium isotope analysis with ionization and inductively coupled plasma multicolector mass spectrometry: high-precision joint measurement of 88Sr/86Sr and 87Sr/86Sr ratio, Mass-spektrometriya, 2012, vol. 9, no. 2, pp. 129–138.
Kroopnick, P.M., The distribution of 13C of ΣCO2 in the world oceans, Deep-Sea Res., 1985, vol. 32, no. 1, pp. 57–84.
Lang, S.Q., Früh-Green, G.L., Bernasconi, S.M., et al., Microbial utilization of abiogenic carbon and hydrogen in a serpentinite-hosted system, Geochim. Cosmochim. Acta, 2012, vol. 92, pp. 82–99.
Lein, A.Yu., Bogdanov, Yu.A., Sagalevich, A.M., et al., A new type of hydrothermal field in the Mid-Atlantic Ridge (Lost City Field, 30° N), Dokl. Earth Sci., 2004, vol. 394, no. 3, pp. 92–95.
Liu, H.C., You, C.F., Huang, K.F., and Chung, C.H., Precise determination of triple Sr isotopes (δ87Sr and δ88Sr) using MC-ICP-MS, Talanta, 2012, vol. 88, pp. 338–344.
Ludwig, K.A., Kelley, D.S., Butterfield, D.A., et al., Formation and evolution of carbonate chimneys at the Lost City hydrothermal field, Geochim. Cosmochim. Acta, 2006, vol. 70, no. 14, pp. 3625–3645.
Millero, F.J., Graham, T.B., Huang, F., et al., Dissociation constants of carbonic acid in seawater as a function of salinity and temperature, Mar. Chem., 2006, vol. 100, nos. 1–2, pp. 80–94.
NOAA Repository. 2005. https://www.nodc.noaa.gov/
Ohmoto, H. and Rye, R.O., Isotope of sulfur and carbon, Geochemestry of Hydrothermal Deposits, H.L. Barnes, Eds., New York: John Wiley & Sons, 1979.
Ohno, T. and Hirata, T., Simultaneous determination of mass-dependent isotopic fractionation and radiogenic isotope variation of strontium in geochemical samples by multiple collector-ICP-mass spectrometry, Anal. Sci., 2007, vol. 23, pp. 1275–1280.
Palandri, J.L. and Reed, M.H., Geochemical models of metasomatism in ultramafic systems: serpentinization, rodingitization, and sea floor carbonate chimney precipitation, Geochim. Cosmochim. Acta, 2004, vol. 68, no. 5, pp. 1115–1133.
Popov, N.I., Fedorov, K.N., and Orlov, V.M., Morskaya voda. Spravochnoe rukovodstvo (Seawater. A Textbook), Moscow: Nauka, 1979.
Proskurowski, G., Lilley, M.D., Kelley, D.S., et al., Low temperature volatile production at the lost city hydrothermal field, evidence from a hydrogen stable isotope geothermometer, Chem. Geol., 2006, vol. 229, pp. 331–343.
Proskurowski, G., Lilley, M.D., Seewald, J.S., et al., Abiogenic hydrocarbon production at lost city hydrothermal field, Science, 2008, vol. 319, pp. 604–607.
Raczek, I., Jochum, K.P., and Hofmann, A.W., Neodymium and strontium isotope data for USGS reference materials BCR-1, BCR-2, BHVO-1, BHVO-2, AGV-1, AGV-2, GSP-1, GSP-2 and eight MPI-DING reference glasses, Geostand. Newslett. J. Geostand. Geoanal., 2003, vol. 27, no. 2, pp. 173–179.
Romanek, C., Grossman, E., and Morse, J., Carbon isotopic fractionation in synthetic calcite, effects of temperature and precipitation rate, Geochim. Cosmochim. Acta, 1992, vol. 56, pp. 419–430.
Shanks, W.S., Stable isotopes in seafloor hydrothermal systems: vent fluids, hydrothermal deposits, hydrothermal alteration, and microbial processes, Stable Isotope Geochemistry.Rev. Mineral., Valley, J.W. and Cole, D.R., Eds., 2001, vol. 43, pp. 469–525.
Silantyev, S.A., Novoselov, A.A., and Mironenko, M.V., hydrothermal systems in peridotites at slow-spreading ridges. modeling phase transformations and material balance: role of gabbroids, Petrology, 2011, vol. 19, no. 3, pp. 217–236.
Silantyev, S.A., Kubrakova, I.V., and Tyutyunnik, O.A., Distribution of siderophile and chalcophile elements in serpentinites of the oceanic lithosphere as an insight into the magmatic and crustal evolution of mantle peridotites, Geochem. Int., 2016, vol. 54, no. 12, pp. 1019–1034.
Teagle, D.A.H., Alt, J.C., and Halliday, A.N., Tracing the chemical evolution of fluids during hydrothermal recharge: constraints from anhydrite recovered in ODP Hole 504b, Earth. Planet. Sci. Lett., 1998, vol. 155, pp. 167–182.
Turekian, K.K. and Wedepohl, K.H., Distribution of the elements in some major units of the earth’s crust, Geology, 1961, vol. 72, pp. 175–182.
Watkins, J.M., Hunt, J.D., Ryerson, F.J., and De Paolo, D.J., The infuence of temperature, pH, and growth rate on the δ18O composition of inorganically precipitated calcite, Earth. Planet. Sci. Lett., 2014, vol. 404, pp. 332–343.
Watson, E.B., A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals, Geochim. Cosmochim. Acta, 2004, vol. 68, no. 7, pp. 1473–1488. www.nodc.noaa. gov/
Zeebe, R.E., An explanation of the effect of seawater carbonate concentration on foraminiferal oxygen isotopes, Geochim. Cosmochim. Acta, 1999, vol. 63, pp. 2001–2007.
Zeebe, R.E., An expression for the overall oxygen isotope fractionation between the sum of dissolved inorganic carbon and water, Geochem. Geophys. Geosyst., 2007, vol. 8, no. 9, p. Q09002. https://doi.org/10.1029/2007GC001663
ACKNOWLEDGMENTS
We are grateful to reviewers for critical comments that improved our manuscript.
Funding
This work was performed in the framework of State Task (project no. 0136-2019-0013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Bogina
Rights and permissions
About this article
Cite this article
Dubinina, E.O., Kramchaninov, A.Y., Silantyev, S.A. et al. Influence of the Precipitation Rate on the Isotope (δ18O, δ13C and δ88Sr) Composition of Carbonate Chimneys of the Lost City Hydrothermal Field (30° N, Mid-Atlantic Ridge). Petrology 28, 374–388 (2020). https://doi.org/10.1134/S0869591120040037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591120040037