Abstract
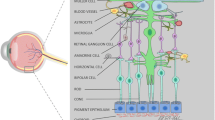
Müller cells are the major type of glial cells in the retina and are actively involved in ensuring its vital functions. They are responsible for maintaining homeostasis and the synaptic activity of neurons by transporting substances to all types of cells within the retina and leading them beyond it. Müller cells guide light to photoreceptors, dividing it according to the wavelength and amplifying the incoming light signal, they protect neurons from damage and are able to regenerate into photoreceptors and other retinal cells under pathological conditions. This review summarizes the knowledge about the morphology and functions of Müller cells which has been accumulated over the years of their study.
Similar content being viewed by others
REFERENCES
Roesch, K., Jadhav, A.P., Trimarchi, J.M., et al., The transcriptome of retinal Müller glial cells, J. Comp. Neurol., 2008, vol. 509, no. 2, p. 225.
Ghai, K., Zelinka, C., and Fischer, A.J., Notch signaling influences neuroprotective and proliferative properties of mature Müller glia, J. Neurosci., 2010, vol. 30, no. 8, p. 3101.
Liu, B., Hunter, D.J., Smith, A.A., et al., The capacity of neural crest-derived stem cells for ocular repair, Birth Defects Res., 2014, vol. 102, no. 3, p. 299.
Reichenbach, A. and Bringmann, A., New functions of Müller cells, Glia, 2013, vol. 61, p. 651.
Vecino, E., Rodriguez, F.D., Ruzafa, N., et al., Glia-neuron interactions in the mammalian retina, Prog. Retinal Eye Res., 2016, vol. 51, p. 1.
Franze, K., Grosche, J., Skatchkov, S.N., et al., Müller cells are living optical fibers in the vertebrate retina, Proc. Natl. Acad. Sci. U.S.A., 2007, vol. 104, no. 20, p. 8287.
Labin, A.M., Safuri, S.K., Ribak, E.N., and Perlman, I., Müller cells separate between wavelengths to improve day vision with minimal effect upon night vision, Nat. Commun., 2014, vol. 5, p. 4319.
Yamada, E. Some structural features of the fovea centralis in the human retina, Arch. Ophthalmol., 1969, vol. 82, p. 151.
Tschulakow, A.V., Oltrup, T., Bende, T., et al., The anatomy of the foveola reinvestigated, PeerJ, 2018, vol. 6, no. 778, p. e4482.
Schubert, H., Cystoid macular edema: the apparent role of mechanical factors, Prog. Clin. Biol. Res., 1989, vol. 312, p. 277.
Lu, Y., Franze, K., Seifert, G., et al., Viscoelastic properties of individual glial cells and neurons in the CNS, Proc. Natl. Acad. Sci. U.S.A., 2006, vol. 103, no. 47, p. 17759.
Lu, Y.-B., Iandiev, I., Hollborn, M., et al., Reactive glial cells: increased stiffness correlates with increased intermediate filament expression, FASEB J., 2011, vol. 25, no. 2, p. 624.
Lundkvist, A., Reichenbach, A., Betsholtz, C., et al., Under stress, the absence of intermediate filaments from Müller cells in the retina has structural and functional consequences, J. Cell Sci., 2004, vol. 117, no. 16, p. 3481.
Lindqvist, N., Liu, Q., Zajadacz, J., et al., Retinal glial (Müller) cells: sensing and responding to tissue stretch, Invest. Ophthalmol. Vis. Sci., 2010, vol. 51, no. 3, p. 1683.
Tian, X., Cheng, Y., Liu, G., et al., Expressions of type I collagen, α2 integrin and β1 integrin in sclera of guinea pig with defocus myopia and inhibitory effects of bFGF on the formation of myopia, Int. J. Ophthalmol., 2013, vol. 6, no. 1, p. 54.
Wu, P., Tsai, C., Wu, H., et al., Outdoor activity during class recess reduces myopia onset and progression in school children, Ophthalmology, 2013, vol. 120, no. 5, p. 1080.
Karouta, C. and Ashby, R.S., Correlation between light levels and the development of deprivation myopia, Invest. Ophthalmol. Vis. Sci., 2014, vol. 56, no. 1, p. 299.
Fu, X., Zhang, X., Xia, W., et al., Effects of 530 nm monochromatic light on basic fibroblast growth factor and transforming growth factor-β1 expression in Müller cells, Int. J. Ophthalmol., 2015, vol. 8, no. 5, p. 904.
Uckermann, O., Vargova, L., Ulbricht, E., et al., Glutamate-evoked alteration of glial and neuronal cell morphology in the guinea pig retina, J. Neurosci., 2004, vol. 24, no. 45, p. 10149.
Dmitriev, A.V., Govardovskii, V.I., Schwahn, H.N., and Steinberg, R.H., Light-induced changes of extracellular ions and volume in the isolated chick retina—pigment epithelium preparation, Vis. Neurosci., 1999, vol. 16, no. 6, p. 1157.
Netti, V., Pizzoni, A., Pérez-Domínguez, M., et al., Release of taurine and glutamate contributes to cell volume regulation in human retinal Müller cells: differences in modulation by calcium, J. Neurophysiol., 2018, vol. 120, no. 3, p. 973.
Reichenbach, A. and Bringmann, A., Müller cells in the healthy and diseased retina, Prog. Retinal Eye Res., 2010, vol. 25, no. 4, p. 397.
Pannicke, T., Iandiev, I., Uckermann, O., et al., A potassium channel-linked mechanism of glial cell swelling in the postischemic retina, Mol. Cell. Neurosci., 2004, vol. 26, no. 4, p. 493.
Pannicke, T., Uckermann, O., Iandiev, I., et al., Ocular inflammation alters swelling and membrane characteristics of rat Müller glial cells, J. Neuroimmunol., 2005, vol. 161, nos. 1–2, p. 145.
Pannicke, T., Iandiev, I., Wurm, A., et al., Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina, Diabetes, 2006, vol. 55, no. 3, p. 633.
Reichenbach, A., Wurm, A., Pannicke, T., et al., Müller cells as players in retinal degeneration and edema, Graefe’s Arch. Clin. Exp. Ophthalmol., 2007, vol. 245, no. 5, p. 627.
Pannicke, T., Wurm, A., Iandiev, I., et al., Deletion of aquaporin-4 renders retinal glial cells more susceptible to osmotic stress, J. Neurosci. Res., 2010, vol. 88, no. 13, p. 2877.
Uckermann, O., Wolf, A., Kutzera, F., et al., Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y, J. Neurosci. Res., 2006, vol. 83, no. 4, p. 538.
Wurm, A., Lipp, S., Pannicke, T., et al., Involvement of A(1) adenosine receptors in osmotic volume regulation of retinal glial cells in mice, Mol. Vis., 2009, vol. 15, p. 1858.
Wurm, A., Lipp, S., Pannicke, T., et al., Endogenous purinergic signaling is required for osmotic volume regulation of retinal glial cells, J. Neurochem., 2010, vol. 112, no. 5, p. 1261.
Yu, J., Chen, C., Wang, J., et al., In vitro effect of adenosine on the mRNA expression of Kir 2.1 and Kir 4.1 channels in rat retinal Müller cells at elevated hydrostatic pressure, Exp. Ther. Med., 2012, vol. 3, no. 4, p. 617.
Bringmann, A., Grosche, A., Pannicke, T., and Reichenbach, A., GABA and glutamate uptake and metabolism in retinal glial (Müller) cells, Front. Endocrinol., 2013, vol. 4, no. 48, p. 1.
Toft-Kehler, A.K., Skytt, D., Poulsen, K., et al., Limited energy supply in Müller cells alters glutamate uptake, Neurochem. Res., 2014, vol. 39, no. 5, p. 941.
Barnett, N.L. and Pow, D.V., Antisense knockdown of GLAST, a glial glutamate transporter, compromises retinal function, Invest. Ophthalmol. Vis. Sci., 2000, vol. 41, no. 2, p. 585.
Bringmann, A., Pannicke, T., Grosche, J., et al., Müller cells in the healthy and diseased retina, Prog. Retinal Eye Res., 2006, vol. 5, no. 4, p. 397.
Chen, H. and Weber, A., Expression of glial fibrillary acidic protein and glutamine synthetase by Müller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factor, Glia, 2002, vol. 38, no. 2, p. 115.
Paasche, G., Gärtner, U., Germer, A., et al., Mitochondria of retinal Müller (glial) cells: the effects of aging and of application of free radical scavengers, Ophthalmic Res., 2000, vol. 32, no. 5, p. 229.
Schütte, M. and Werner, P., Redistribution of glutathione in the ischemic rat retina, Neurosci. Lett., 1998, vol. 246, no. 1, p. 53.
Brückner, E., Grosche, A., Pannicke, T., et al., Mechanisms of VEGF- and glutamate-induced inhibition of osmotic swelling of murine retinal glial (Müller) cells: indications for the involvement of vesicular glutamate release and connexin-mediated ATP release, Neurochem. Res., 2012, vol. 37, no. 2, p. 268.
Powner, M.B., Gillies, M.C., Zhu, M., et al., Loss of Müller’s cells and photoreceptors in macular telangiectasia type 2, Ophthalmology, 2013, vol. 120, no. 11, p. 2344.
Bringmann, A., Iandiev, I., Pannicke, T., et al., Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects, Prog. Retinal Eye Res., 2009, vol. 28, no. 6, p. 423.
Shen, W., Fruttiger, M., Zhu, L., et al., Conditional Müller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model, J. Neurosci., 2012, vol. 32, no. 45, p. 15715.
Jablonski, M. and Iannaccone, A., Targeted disruption of Müller cell metabolism induces photoreceptor dysmorphogenesis, Glia, 2000, vol. 32, no. 2, p. 192.
Wang, J. and Kefalov, V.J., The cone-specific visual cycle, Prog. Retinal Eye Res., 2011, vol. 30, no. 2, p. 115.
Wang, J., Estevez, M., Cornwall, M., and Kefalov, V., Intra-retinal visual cycle required for rapid and complete cone dark adaptation, Nat. Neurosci., 2009, vol. 12, no. 3, p. 295.
Tout, S., Chan-Ling, T., Holländer, H., and Stone, J., The role of Müller cells in the formation of the blood-retinal barrier, Neuroscience, 1993, vol. 55, no. 1, p. 291.
Eichler, W., Yafai, Y., Keller, T., et al., PEDF derived from glial Müller cells: a possible regulator of retinal angiogenesis, Exp. Cell. Res., 2004, vol. 299, no. 1, p. 68.
Eichler, W., Yafai, Y., Wiedemann, P., and Reichenbach, A., Angiogenesis-related factors derived from retinal glial (Müller) cells in hypoxia, Neuroreport, 2004, vol. 15, no. 10, p. 1633.
Fausett, B.V. and Goldman, D., A role for α1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina, J. Neurosci., 2006, vol. 26, no. 23, p. 6303.
Lindsey, A.E. and Powers, M.K., Visual behavior of adult goldfish with regenerating retina, Vis. Neurosci., 2007, vol. 24, no. 3, p. 247.
Goldman, D., Müller glia cell reprogramming and retina regeneration, Nat. Rev. Neurosci., 2014, vol. 15, no. 7, p. 431.
Powell, C., Grant, A.R., Cornblath, E., and Goldman, D., Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration, Proc. Natl. Acad. Sci. U.S.A., 2013, vol. 110, no. 49, p. 19814.
Nagashima, M., Barthel, L.K., Raymond, P.A. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons, Development, 2013, vol. 140, no. 22, p. 4510.
Ramachandran, R., Reifler, A., Parent, J., et al., Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration, J. Comp. Neurol., 2010, vol. 518, no. 20, p. 4196.
Fischer, A. and Reh, T., Müller glia are a potential source of neural regeneration in the postnatal chicken retina, Nat. Neurosci., 2001, vol. 4, no. 3, p. 247.
Jadhav, A.P., Roesch, K., and Cepko, C.L., Development and neurogenic potential of Müller glial cells in the vertebrate retina, Prog. Retinal Eye Res., 2011, vol. 28, no. 4, p. 249.
Jorstad, N., Wilken, M., Grimes, W., et al., Stimulation of functional neuronal regeneration from Müller glia in adult mice, Nature, 2017, vol. 548, no. 7665, p. 103.
Yao, K., Qiu, S., Tian, L., et al., Wnt regulates proliferation and neurogenic potential of Müller glial cells via a Lin28/let-7 miRNA-dependent pathway in adult mammalian retinas, Cell Rep., 2016, vol. 17, no. 1, p. 165.
Yao, K., Qiu, S., Wang,Y. V., et al., Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas, Nature, 2018, vol. 560, no. 7719, p. 484.
Jayaram, H., Jones, M., Eastlake, K., et al., Transplantation of photoreceptors derived from human Müller glia restore rod function in the P23H rat, Stem Cells Transl. Med., 2014, vol. 3, no. 3, p. 323.
Singhal, S., Bhatia, B., Jayaram, H., et al., Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation, Stem Cells Transl. Med., 2012, vol. 1, no. 3, p. 188.
Rueda, E.M., Hall, B.M., Hill, M.C., et al., The hippo pathway blocks mammalian retinal Müller glial cell reprogramming, Cell Rep., 2019, vol. 27, no. 6, p. 1637.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tikhonovich, M.V., Gavrilova, S.A. & Ioshin, I.E. Müller Cells: Genii Loci. Hum Physiol 46, 696–702 (2020). https://doi.org/10.1134/S0362119720050126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119720050126