Abstract
Background
Under normal conditions, Müller cells support neuronal activity and the integrity of the blood-retinal barrier, whereas gliotic alterations of Müller cells under pathological conditions may contribute to retinal degeneration and edema formation. A major function of Müller cells is the fluid absorption from the retinal tissue, which is mediated by transcellular water transport coupled to currents through potassium channels.
Methods
Alterations of retinal Müller cells under pathological conditions were investigated by immunohistochemistry and recording their behavior under osmotic stress.
Results
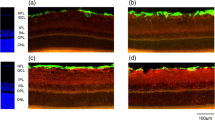
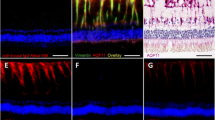
In animal models of various retinopathies, e.g., retinal ischemia, ocular inflammation, retinal detachment, and diabetes, it was found that Müller cells decrease the expression of their major potassium channel (Kir4.1). This alteration is associated with an impairment of the rapid water transport across Müller cell membranes, as recognizable in the induction of cellular swelling under hypoosmolar conditions. Osmotic swelling of Müller cells is also induced by oxidative stress and by inflammatory mediators such as arachidonic acid and prostaglandins.
Conclusions
The data suggest that a disturbed fluid transport through Müller cells is (in addition to vascular leakage) a pathogenic factor contributing to the development of retinal edema. Pharmacological re-activation of the retinal water clearance by Müller cells may represent an approach to the development of new edema-resolving drugs. Triamcinolone acetonide, which is clinically used to resolve edema, prevents osmotic swelling of Müller cells as it induces the release of endogenous adenosine and subsequent A1 receptor activation which results in the opening of ion channels. Apparently, triamcinolone resolves edema by both inhibition of vascular leakage and stimulation of retinal fluid clearance by Müller cells.




Similar content being viewed by others
References
Ferris FL, Patz A (1984) Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 28:S452–S461
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A (1996) Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 80:332–336
Ray S, D’Amico DJ (2002) Pseudophakic cystoid macular edema. Semin Ophthalmol 17:167–180
Bresnick GH (1983) Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology 90:1301–1317
Guex-Crosier Y (1999) The pathogenesis and clinical presentation of macular edema in inflammatory diseases. Doc Ophthalmol 97:297–309
van Dam PS (2002) Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 18:176–184
Miyake K, Ibaraki N (2002) Prostaglandins and cystoid macular edema. Surv Ophthalmol 47:S203–S218
Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW (2005) Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets 6:511–524
Speicher MA, Danis RP, Criswell M, Pratt L (2003) Pharmacologic therapy for diabetic retinopathy. Expert Opin Emerg Drugs 8:239–250
Eichler W, Yafai Y, Wiedemann P, Fengler D (2006) Antineovascular agents in the treatment of eye diseases. Curr Pharm Des 12:2645–2660
Cunha-Vaz JG, Travassos A (1984) Breakdown of the blood-retinal barriers and cystoid macular edema. Surv Ophthalmol 28:S485–S492
Derevjanik NL, Vinores SA, Xiao WH, Mori K, Turon T, Hudish T, Dong S, Campochiaro PA (2002) Quantitative assessment of the integrity of the blood-retinal barrier in mice. Invest Ophthalmol Vis Sci 43:2462–2467
Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL (1997) Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 46:1473–1480
Antcliff RJ, Marshall J (1999) The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol 14:223–232
Marmor MF (1999) Mechanisms of fluid accumulation in retinal edema. Doc Ophthalmol 97:239–249
Lobo CL, Bernardes RC, Cunha-Vaz JG (2000) Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch Ophthalmol 118:1364–1369
Mori F, Hikichi T, Takahashi J, Nagaoka T, Yoshida A (2002) Dysfunction of active transport of blood-retinal barrier in patients with clinically significant macular edema in type 2 diabetes. Diabetes Care 25:1248–1249
Bellhorn RW (1984) Analysis of animal models of macular edema. Surv Ophthalmol 28:S520–S524
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A (2006) Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25:397–424
Lieth E, Barber A, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM (1998) Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes 47:815–820
Rungger-Brändle E, Dosso AA, Leuenberger PM (2000) Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 4:1971–1980
Tout S, Chan-Ling T, Hollander H, Stone J (1993) The role of Müller cells in the formation of the blood-retinal barrier. Neuroscience 55:291–301
Tretiach M, Madigan MC, Wen L, Gillies MC (2005) Effect of Müller cell co-culture on in vitro permeability of bovine retinal vascular endothelium in normoxic and hypoxic conditions. Neurosci Lett 378:160–165
Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA (1995) Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 113:1538–1544
Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW (1997) Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 38:36–47
Eichler W, Kuhrt H, Hoffmann S, Wiedemann P, Reichenbach A (2000) VEGF release by retinal glia depends on both oxygen and glucose supply. Neuroreport 11:3533–3537
Yafai Y, Iandiev I, Wiedemann P, Reichenbach A, Eichler W (2004) Retinal endothelial angiogenic activity: effects of hypoxia and glial (Müller) cells. Microcirculation 11:577–586
Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB (2001) TGF-ß increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci 42:853–859
Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A (2003) P2Y receptor-mediated stimulation of Müller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci 44:1211–1220
Giebel SJ, Menicucci G, McGuire PG, Das A (2005) Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest 85:597–607
Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A (2004) PEDF derived from glial Müller cells: a possible regulator of retinal angiogenesis. Exp Cell Res 299:68–78
Duh EJ, Yang HS, Suzuma I, Miyagi M, Youngman E, Mori K, Katai M, Yan L, Suzuma K, West K, Davarya S, Tong P, Gehlbach P, Pearlman J, Crabb JW, Aiello LP, Campochiaro PA, Zack DJ (2002) Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci 43:821–829
Fine BS, Brucker AJ (1981) Macular edema and cystoid macular edema. Am J Ophthalmol 92:466–481
Yanoff M, Fine BS, Brucker AJ, Eagle RC (1984) Pathology of human cystoid macular edema. Surv Ophthalmol 28:S505–S511
Gass JD, Anderson DR, Davis EB (1985) A clinical, fluorescein angiographic, and electron microscopic correlation of cystoid macular edema. Am J Ophthalmol 100:82–86
Kimelberg HK (1995) Current concepts of brain edema. Review of laboratory investigations. J Neurosurg 83:1051–1059
Loeffler KU, Li ZL, Fishman GA, Tso MOM (1992) Dominantly inherited cystoid macular edema. A histopathologic study. Ophthalmology 99:1385–1392
Stepinac TK, Chamot SR, Rungger-Brändle E, Ferrez P, Munoz JL, van den Bergh H, Riva CE, Pournaras CJ, Wagnieres GA (2005) Light-induced retinal vascular damage by Pd-porphyrin luminescent oxygen probes. Invest Ophthalmol Vis Sci 46:956–966
Pederson JE (1994) Fluid physiology of the subretinal space. In: Wilkinson CP (ed) Retina. Mosby, St. Louis, pp 1955–1968
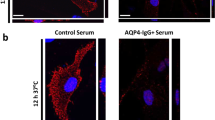
Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, Kurachi Y, Ottersen OP (1999) Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 26:47–54
Verkman AS (2002) Physiological importance of aquaporin water channels. Ann Med 34:192–200
Manley GT, Binder DK, Papadopoulos MC, Verkman AS (2004) New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience 129:983–991
Stamer WD, Bok D, Hu J, Jaffe GJ, McKay BS (2003) Aquaporin-1 channels in human retinal pigment epithelium: role in transepithelial water movement. Invest Ophthalmol Vis Sci 44:2803–2808
Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP (1998) Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J Neurosci 18:2506–2519
Bialek S, Miller SS (1994) K+ and Cl− transport mechanisms in bovine pigment epithelium that could modulate subretinal space volume and composition. J Physiol (Lond) 475:401–417
Newman EA, Reichenbach A (1996) The Müller cell: a functional element of the retina. Trends Neurosci 19:307–312
Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, Thomzig A, Veh RW, Bringmann A, Reichenbach A (2002) Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia 39:292–303
Iandiev I, Tenckhoff S, Pannicke T, Biedermann B, Hollborn M, Wiedemann P, Reichenbach A, Bringmann A (2006) Differential regulation of Kir4.1 and Kir2.1 expression in the ischemic rat retina. Neurosci Lett 396:97–101
Pannicke T, Iandiev I, Uckermann O, Biedermann B, Kutzera F, Wiedemann P, Wolburg H, Reichenbach A, Bringmann A (2004) A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol Cell Neurosci 26:493–502
Pannicke T, Uckermann O, Iandiev I, Biedermann B, Wiedemann P, Perlman I, Reichenbach A, Bringmann A (2005) Altered membrane physiology in Müller glial cells after transient ischemia of the rat retina. Glia 50:1–11
Pannicke T, Uckermann O, Iandiev I, Wiedemann P, Reichenbach A, Bringmann A (2005) Ocular inflammation alters swelling and membrane characteristics of rat Müller glial cells. J Neuroimmunol 161:145–154
Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes H-P, Bringmann A (2006) Diabetes alters osmotic-swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes 55:633–639
Iandiev I, Uckermann O, Pannicke T, Wurm A, Tenckhoff S, Pietsch UC, Reichenbach A, Wiedemann P, Bringmann A, Uhlmann S (2006) Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci 47:2161–2171
Wurm A, Pannicke T, Iandiev I, Bühner E, Pietsch U-C, Reichenbach A, Wiedemann P, Uhlmann S, Bringmann A (2006) Changes in membrane conductance play a pathogenic role in osmotic glial cell swelling in detached retinas. Am J Pathol 169:1990–1998
Dalloz C, Sarig R, Fort P, Yaffe D, Bordais A, Pannicke T, Grosche J, Mornet D, Reichenbach A, Sahel J, Nudel U, Rendon A (2003) Targeted inactivation of dystrophin gene product Dp71: phenotypic impact in mouse retina. Hum Mol Genet 12:1543–1554
Bringmann A, Pannicke T, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A (2002) Membrane conductance of Müller glial cells in proliferative diabetic retinopathy. Can J Ophthalmol 37:221–227
Wurm A, Pannicke T, Iandiev I, Wiedemann P, Reichenbach A, Bringmann A (2006) The developmental expression of K+ channels in retinal glial cells is associated with a decrease of osmotic cell swelling. Glia 54:411–423
Uckermann O, Kutzera F, Wolf A, Pannicke T, Reichenbach A, Wiedemann P, Wolf S, Bringmann A (2005) The glucocorticoid triamcinolone acetonide inhibits osmotic swelling of retinal glial cells via stimulation of endogenous adenosine signaling. J Pharmacol Exp Ther 315:1036–1045
Kowluru RA, Kennedy A (2001) Therapeutic potential of anti-oxidants and diabetic retinopathy. Expert Opin Investig Drugs 10:1665–1676
Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP (2002) Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-α suppression. FASEB J 16:438–440
Santilli F, Cipollone F, Mezzetti A, Chiarelli F (2004) The role of nitric oxide in the development of diabetic angiopathy. Horm Metab Res 36:319–335
Baydas G, Tuzcu M, Yasar A, Baydas B (2004) Early changes in glial reactivity and lipid peroxidation in diabetic rat retina: effects of melatonin. Acta Diabetol 41:123–128
Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP (2004) A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 18:1450–1452
Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P (2005) Expression of acute-phase response proteins in retinal Müller cells in diabetes. Invest Ophthalmol Vis Sci 46:349–357
Leal EC, Santiago AR, Ambrosio AF (2005) Old and new drug targets in diabetic retinopathy: from biochemical changes to inflammation and neurodegeneration. Curr Drug Targets CNS Neurol Disord 4:421–434
Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP (2001) Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 158:147–152
Du Y, Sarthy VP, Kern TS (2004) Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol 287:R735–R741
Stefánsson E, Wilson CA, Lightman SL, Kuwabara T, Palestine AG, Wagner HG (1987) Quantitative measurements of retinal edema by specific gravity determinations. Invest Ophthalmol Vis Sci 28:1281–1289
Szabo ME, Droy-Lefaix MT, Doly M, Carré C, Braquet P (1991) Ischemia and reperfusion-induced histologic changes in the rat retina. Invest Ophthalmol Vis Sci 32:1471–1478
Davidge ST, Baker PN, Laughlin MK, Roberts JM (1995) Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res 77:274–283
Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ (1996) Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci USA 93:15069–15074
Rehncrona S, Westerberg E, Akesson B, Siesjö BK (1982) Brain cortical fatty acid and phospholipids during and following complete and severe incomplete ischemia. J Neurochem 38:84–93
Yoshida S, Ikeda M, Busto R, Santiso M, Martinez E, Ginsberg M (1986) Cerebral phosphoinositide, triacylglycerol and energy metabolism in reversible ischemia: origin and fate of free fatty acids. J Neurochem 47:744–757
Abe K, Kogure K, Yamamoto H, Imazawa M, Miyamoto K (1987) Mechanism of arachidonic acid liberation during ischemia in gerbil cerebral cortex. J Neurochem 48:503–509
Umemura A, Mabe H, Nagai H, Sugino F (1992) Action of phospholipase A2 and C on free fatty acid release during complete ischemia in rat neocortex. Effect of phospholipase C inhibitor and N-methyl-D-aspartate antagonist. J Neurosurg 76:648–651
Asano T, Shigeno T, Johshita H, Usui M, Hanamura T (1987) A novel concept on the pathogenetic mechanism underlying ischaemic brain oedema: relevance of free radicals and eicosanoids. Acta Neurochir Suppl (Wien) 41:85–96
Chan PH, Fishman RA, Caronna J, Schmidley JW, Prioleau G, Lee J (1983) Induction of brain edema following intracerebral injection of arachidonic acid. Ann Neurol 13:625–632
Wahl M, Schilling L, Unterberg A, Baethmann A (1993) Mediators of vascular and parenchymal mechanisms in secondary brain damage. Acta Neurochir Suppl (Wien) 57:64–72
Staub F, Winkler A, Peters J, Kempski O, Kachel V, Baethmann A (1994) Swelling, acidosis, and irreversible damage of glial cells from exposure to arachidonic acid in vitro. J Cereb Blood Flow Metab 14:1030–1039
Lees GJ (1991) Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Rev 16:283–380
Owada S, Larsson O, Arkhammar P, Katz AI, Chibalin AV, Berggren PO, Bertorello AM (1999) Glucose decreases Na,K-ATPase activity in pancreatic ß-cells: an effect mediated via Ca2+-independent phospholipase A2 and protein kinase C-dependent phosphorylation of the α-subunit. J Biol Chem 274:2000–2008
Uckermann O, Wolf A, Kutzera F, Kalisch F, Beck-Sickinger AG, Wiedemann P, Reichenbach A, Bringmann A (2006) Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res 83:538–550
Bringmann A, Skatchkov SN, Biedermann B, Faude F, Reichenbach A (1998) Alterations of potassium channel activity in retinal Müller glial cells induced by arachidonic acid. Neuroscience 86:1291–1306
Chao TI, Henke A, Reichelt W, Eberhardt W, Reinhardt-Maelicke S, Reichenbach A (1994) Three distinct types of voltage-dependent K+ channels are expressed by Müller (glial) cells of the rabbit retina. Pflügers Arch 426:51–60
Francke M, Pannicke T, Biedermann B, Faude F, Wiedemann P, Reichenbach A, Reichelt W (1997) Loss of inwardly rectifying potassium currents by human retinal glial cells in diseases of the eye. Glia 20:210–218
Bringmann A, Francke M, Pannicke T, Biedermann B, Faude F, Enzmann V, Wiedemann P, Reichelt W, Reichenbach A (1999) Human Müller glial cells: altered potassium channel activity in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 40:3316–3323
Sanchez-Olea R, Morales-Mulia M, Moran J, Pasantes-Morales H (1995) Inhibition by polyunsaturated fatty acids of cell volume regulation and osmolyte fluxes in astrocytes. Am J Physiol 269:C96–C102
Aiello LP (2002) The potential role of PKC beta in diabetic retinopathy and macular edema. Surv Ophthalmol 47:S263–S269
Sakamoto T, Miyazaki M, Hisatomi T, Nakamura T, Ueno A, Itaya K, Ishibashi T (2002) Triamcinolone-assisted pars plana vitrectomy improves the surgical procedures and decreases the postoperative blood-ocular barrier breakdown. Graefes Arch Clin Exp Ophthalmol 240:423–429
Edelman JL, Lutz D, Castro MR (2005) Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res 80:249–258
Brooks HL Jr, Caballero S Jr, Newell CK, Steinmetz RL, Watson D, Segal MS, Harrison JK, Scott EW, Grant MB (2004) Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol 122:1801–1807
Sears JE, Hoppe G (2005) Triamcinolone acetonide destabilizes VEGF mRNA in Müller cells under continuous cobalt stimulation. Invest Ophthalmol Vis Sci 46:4336–4341
Matsuda S, Gomi F, Oshima Y, Tohyama M, Tano Y (2005) Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE19 cells under oxidative stress. Invest Ophthalmol Vis Sci 46:1062–1068
Itakura H, Akiyama H, Hagimura N, Doi H, Tanaka T, Kishi S, Kurabayashi M (2006) Triamcinolone acetonide suppresses interleukin-1 beta-mediated increase in vascular endothelial growth factor expression in cultured rat Müller cells. Graefes Arch Clin Exp Ophthalmol 244:226–231
Maminishkis A, Jalickee S, Blaug SA, Rymer J, Yerxa BR, Peterson WM, Miller SS (2002) The P2Y2 receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci 43:3555–3566
Meyer CH, Hotta K, Peterson WM, Toth CA, Jaffe GJ (2002) The effects of INS37217, a P2Y2 receptor agonist, on experimental retinal detachment and electroretinogram in adult rabbits. Invest Ophthalmol Vis Sci 43:3567–3574
Skatchkov SN, Eaton MJ, Shuba YM, Kucheryavykh YV, Derst C, Veh RW, Wurm A, Iandiev I, Pannicke T, Bringmann A, Reichenbach A (2006) Tandem-pore domain potassium channels are functionally expressed in retinal (Müller) glial cells. Glia 53:266–276
Larsen AK, Osborne NN (1996) Involvement of adenosine in retinal ischemia. Studies on the rat. Invest Ophthalmol Vis Sci 37:2603–2611
Ghiardi GJ, Gidday JM, Roth S (1999) The purine nucleoside adenosine in retinal ischemia-reperfusion injury. Vision Res 39:2519–2535
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW (2002) Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 47:S253–S262
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (GRK 1097/1) and from the Interdisziplinäres Zentrum für Klinische Forschung (IZKF) at the Faculty of Medicine of the University of Leipzig (C21, Z10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichenbach, A., Wurm, A., Pannicke, T. et al. Müller cells as players in retinal degeneration and edema. Graefe's Arch Clin Exp Ophthalmol 245, 627–636 (2007). https://doi.org/10.1007/s00417-006-0516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-006-0516-y