Abstract
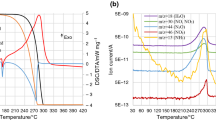
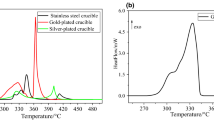
In this study, the thermal decomposition kinetics of ammonium nitrate (AN) under nonisothermal conditions in an open system at different heating rates has been examined using thermogravimetry and differential scanning calorimetry. An equation for the kinetics of the thermal decomposition of AN in an open system has been proposed based on the experiments.





Similar content being viewed by others
REFERENCES
Volkova, A.V., Rynok mineral’nykh udobrenii. I kvartal 2017 goda (The Market of Mineral Fertilizers: The First Quarter of 2017), Moscow: Tsentr Razvit., Nats. Issled. Univ. Vyssh. Shk. Ekon., 2017.
Djerjiev, A.M., Priyananda, P., Gore, J., Beattie, J.K., Neto, C., and Hawkett, B.S., The mechanism of the spontaneous detonation of ammonium nitrate in reactive grounds, J. Environ. Chem. Eng., 2018, vol. 1, no. 6, pp. 281–288.
Chaturvedi, S. and Dave, P.N., Review on thermal decomposition of ammonium nitrate, J. Energ. Mater., 2016, vol. 31, pp. 1–26.
Marlair, G., Michit, C., Turcotte, R., and Singh, S., Comments about the paper entitled “Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years” by Pittman et al. (J. Hazard. Mater. 280 (2014), 472–477), J. Hazard. Mater., 2016, vol. 303, pp. 177–180.
Vyazovkin, S., Chrissafis, K., Di Lorenzo, M.L., Koga, N., Pijolat, M., Roduit, B., Sbirrazzuoli, N., and Sunol, J.J., ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations, Thermochim. Acta, 2014, vol. 590, pp. 1–23.
Farjas, J., Butchosa, N., and Roura, P., A simple kinetic method for the determination of the reaction model from non-isothermal experiments, J. Therm. Anal. Calorim., 2010, vol. 102, pp. 615–625.
Sbirrazzuoli, N., Determination of pre-exponential factors and of the mathematical functions f (α) or G(α) that describe the reaction mechanism in a model-free way, Thermochim. Acta, 2013, vol. 564, pp. 59–69.
Herrmann, M.J. and Engel, W., Phase transition and lattice dynamics of ammonium nitrate, Propellants, Explos., Pyrotech., 1997, vol. 22, pp. 143–147.
Sorescu, D.C. and Thompson, D.L., Classical and quantum mechanical studies of crystalline ammonium nitrate, J. Phys. Chem. A, 2001, vol. 105, pp. 720–733.
Dunuwille, M. and Yoo, C.-S., Phase diagram of ammonium nitrate, J. Chem. Phys., 2013, vol. 139, no. 214503, pp. 1–11.
Izato, Y. and Miyake, A., Thermal decomposition of molten ammonium nitrate (AN). Chemical equilibrium in molten AN, J. Therm. Anal. Calorim., 2015, vol. 122, no. 2, pp. 595–600.
Manelis, G.B., Nazin, G.M., Rubtsov, Yu.I., and Strunin, V.A., Termicheskoe razlozhenie i gorenie vzryvchatykh veshchestv i porokhov (Thermal Decomposition and Combustion of Explosives and Blasting Powders), Moscow: Nauka, 1996.
Hildenbrand, D.L., Lau, K.H., and Chandra, D., Revised thermochemistry of gaseous ammonium nitrate, NH4NO3(g), J. Phys. Chem. A, 2010, vol. 114, pp. 11654–11655.
Irikura, K.K., Thermochemistry of ammonium nitrate, NH4NO3, in the gas phase, J. Phys. Chem. A, 2010, vol. 114, pp. 11651–11653.
Kazakov, A.I., Andrienko, L.P., and Rubtsov, Yu.I., Kinetics and mechanism of oxidation of the ammonium ion by nitric acid solution, Russ. Chem. Bull., 1980, vol. 29, pp. 681–685.
Yang, M., Chen, X., Wang, Y., Yuan, B., Niu, Y., Zhang, Y., Liao, R., and Zhang, Z., Comparative evaluation of thermal decomposition behavior and thermal stability of powdered ammonium nitrate under different atmosphere conditions, J. Hazard. Mater., 2017, vol. 337, pp. 10–19.
Izato, Y. and Miyake, A., Kinetic analysis of the thermal decomposition of liquid ammonium nitrate based on thermal analysis and detailed reaction simulations, J. Therm. Anal. Calorim., 2018, vol. 134, no. 1, pp. 813–823.
Han, Z., Sachdeva, S., Papadaki, M., and Mannan, M.S., Calorimetry studies of ammonium nitrate – Effect of inhibitors, confinement, and heating rate, J. Loss Prev. Process Ind., 2015, vol. 38, pp. 234–242.
Vyazovkin, S., Clawson, J.S., and Wight, C.A., Thermal dissociation kinetics of solid and liquid ammonium nitrate, Chem. Mater., 2001, vol. 13, no. 3, pp. 960–966.
Gunawan, R. and Zhang, D., Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite, J. Hazard. Mater., 2010, vol. 165, pp. 751–758.
Cao, H.-Q., Jiang, L., Duan, Q.-L., Zhang, D., Chen, H.D., and Sun, J.-H., An experimental and theoretical study of optimized selection and model reconstruction for ammonium nitrate pyrolysis, J. Hazard. Mater., 2019, vol. 364, pp. 539–547.
Funding
A.I. Kazakov is grateful for financial support from state research grant no. 0089-2019-0005 (registration number no. АААА-А19-119101500098-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Kharitonov
Rights and permissions
About this article
Cite this article
Gorbovskiy, K.G., Kazakov, A.I., Norov, A.M. et al. Ammonium Nitrate Thermal Decomposition Kinetics under Nonisothermal Conditions in Open System. Theor Found Chem Eng 55, 742–747 (2021). https://doi.org/10.1134/S0040579521040084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521040084