Abstract
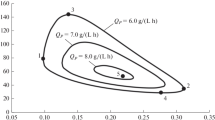
The results of an analysis of a generalized mathematical model for a continuous fermentation process for lactic acid production have been presented. The mathematical model includes a system of equations for the material balance of the main substrate (S), the substrate produced from raw materials during fermentation (M), biomass (X), a product (P), and a by-product (B). The kinetics of the formation of biomass (the equation for the specific rate μ) takes into account inhibition by the substrate, biomass, and product. An analysis has been performed from the standpoint of the possibility of estimating technological characteristics at a given dilution rate D (D = v/V, where v is the volumetric flow rate through a fermenter, m3/h, and V is the volume of the fermenter, m3). The limiting values of \(S{\kern 1pt} '\) and D that ensure the practical implementation of the process have been estimated (\(S{\kern 1pt} '\) includes the initial concentrations of the main substrate S0 and the component that produces the substrate during synthesis M0). These characteristics have been designated as coordinates of “singular points.” The coordinates of a point that corresponds to the maximum productivity with respect to lactic acid QP (\({{Q}_{P}} = DP,\) where P is the concentration of lactic acid) have been determined simultaneously. Relationships have been derived for calculating sets of technological characteristics (the initial characteristics D, S0, and M0 and the current characteristics X, S, P, B, and M) based on a given value of D within permissible limits. It has been shown that, for the values of D that correspond to singular points (the first, second, and optimal points), there is one set and, for the other values of D, there are two sets. Numerical estimates of technological characteristics for the values of constants that correspond to the basic variant have been given. It has been shown that, with an increase in the productivity QP while approaching the value of max QP, the region of estimates of technological characteristics narrows. It has been recommended that the results of this study be used to predict the economic estimates of the implementation of particular conditions of a technological process.
Similar content being viewed by others
REFERENCES
Kumar, G.P., Sastry, I.V.K.S., and Chidambaram, M., Periodic operation of a bioreactor with input multiplicities, Can. J. Chem. Eng., 1993, vol. 71, no. 5, pp. 766 –770. https://doi.org/10.1002/cjce.5450710515
Gordeeva, Yu.L., Ivashkin, Yu.A., and Gordeev, L.S., Steady states of a biotechnological process for producing lactic acid at a given dilution rate, Theor. Found. Chem. Eng., 2013, vol. 47, no. 2, pp. 149–152. https://doi.org/10.1134/S0040579513020036
Gordeeva, Yu.L. and Gordeev, L.S., Steady states of a biotechnological process for producing lactic acid at a given substrate concentration in the inlet stream, Theor. Found. Chem. Eng., 2014, vol. 48, no. 3, pp. 262–266. https://doi.org/10.1134/S0040579514030063
Bouguettoucha, A., Balannec, B., and Amrane, A., Unstructured models for lactic acid fermentation—A review, Food Technol. Biotechnol., 2011, vol. 49, no. 1, pp. 3–12.
Abdel-Rahman, M.A., Tashiro, Y., and Sonomoto, K., Recent advances in lactic acid production by microbial fermentation processes, Biotechnol. Adv., 2013, vol. 31, no. 6, pp. 877–902. https://doi.org/10.1016/j.biotechadv.2013.04.002
Åkerberg, C., Hofvendahl, K., Zacchi, G., and Hahn-Hägerdal, B., Modelling the influence of pH, temperature, glucose and lactic acid concentrations on the kinetics of lactic acid production by Lactococcus lactis ssp. lactis ATCC 19435 in whole-wheat flour, Appl. Microbiol. Biotechnol., 1998, vol. 49, no. 6, pp. 682–690. https://doi.org/10.1007/s002530051232
Gonzalez, K., Tebbani, S., Lopes, F., Thorigné, A., Givry, S., Dumur, D., and Pareau, D., Modeling the continuous lactic acid production process from wheat flour, Appl. Microbiol. Biotechnol., 2016, vol. 100, no. 1, pp. 147–159. https://doi.org/10.1007/s00253-015-6949-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Uteshinsky
Appendices
APPENDIX
The maximum limiting value of the dilution rate is as follows:
The equations for calculating max QP (the maximum value of QP) and the corresponding value of the dilution rate have the following form:
NOTATION
B | concentration of the total number of by-products, g/L |
D | dilution rate, h–1 |
K i | inhibition constant, g/L |
K m | substrate saturation constant, g/L |
k M | constant that determines the amount of produced substrate, h–1 |
M | concentration of raw materials that additionally produce the substrate, g/L |
P | concentration of the product, g/L |
Q P | productivity, g/(L h) |
S | concentration of the substrate, g/L |
X | concentration of biomass, g/L |
Y X/S | stoichiometric coefficient, g/g |
α, αB, β, βB | constants |
μ | specific rate of microorganism growth, h–1 |
SUBSCRIPTS AND SUPERSCRIPTS
0 | initial value |
lim | limiting value |
max | maximum value |
opt | optimum value |
Rights and permissions
About this article
Cite this article
Gordeeva, Y.L., Borodkin, A.G. & Gordeeva, E.L. Steady States of a Continuous Fermentation Process for Lactic Acid Production: The Multiplicity for a Given Dilution Rate. Theor Found Chem Eng 54, 482–488 (2020). https://doi.org/10.1134/S0040579520020062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520020062