Abstract
A kinetic model of the simultaneous saccharification, protein hydrolysis, and fermentation (SSPHF) process for lactic acid production from wheat flour has been developed. The model describes the bacterial growth, substrate consumption, lactic acid production, and maltose hydrolysis. The model was fitted and validated with data from SSPHF experiments obtained under different dilution rates. The results of the model are in good agreement with the experimental data. Steady state concentrations of biomass, lactic acid, glucose, and maltose as function of the dilution rate were predicted by the model. This steady state analysis is further useful to determine the operating conditions that maximize lactic acid productivity.






Similar content being viewed by others
References
Abdel-Rahman M, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902. doi:10.1016/j.biotechadv.2013.04.002
Akerberg C, Hofvendahl K, Zacchi C, Hahn-Hägerdal B (1998) Modelling the influence of pH, temperature, glucose and lactic acid concentrations on the kinetics of lactic acid production by Lactococcus lactis ssp. lactis ATCC 19435 in whole-wheat flour. Appl Microbiol Biotechnol 49:682–690. doi:10.1007/s002530051232
Amrane A (2001) Batch cultures of supplemented whey permeate using Lactobacillus helveticus: unstructured model for biomass formation, substrate consumption and lactic acid production, enzyme microb. Technol 28:827–834. doi:10.1016/S0141-0229(01)00341-6
Anuradha R, Suresh AK, Venkatesh KV (1999) Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochem 35:367–375. doi:10.1016/S0032-9595(99)00080-1
Ben Youssef C, Guillou V, Olmos-Dichara A (2000) Modeling and adaptive control strategy in a lactic fermentation process. Control Eng Pract 8:1297–1307. doi:10.1016/S0967-0661(00)00061-7
Boonmee M, Leksawasdi N, Bridge W, Rogers PL (2003) Batch and continuous culture of Lactococcus lactis NZ133: experimental data and model development. Biochem Eng J 14:127–135. doi:10.1016/S1369-703X(02)00171-7
Bouguettoucha A, Nacef S, Balannec B, Amrane A (2009) Unstructured models for growth and lactic acid production during two-stage continuous cultures of Lactobacillus helveticus. Process Biochem 44:742–748. doi:10.1016/j.procbio.2009.03.010
Bouguettoucha A, Balannec B, Amrane A (2011) Unstructured models for lactic acid fermentation. Food Technol Biotechnol 49(1):3–12
Dey P, Pal P (2013) Modelling and simulation of continuous L(+) lactic acid production from sugarcane juice in membrane integrated hybrid-reactor system. Biochem Eng J 79:15–24. doi:10.1016/j.bej.2013.06.014
Dutta SK, Mukherjee A, Chakraborty P (1996) Effect of product inhibition on lactic acid fermentation: simulation and modelling. Appl Microbiol Biotechnol 46:410–413. doi:10.1007/BF00166238
Fletcher R (1987) Practical methods of optimization. Wiley, Chichester
Friedman M (2004) Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem 52:385–406. doi:10.1021/jf030490p
Gonzalez K, Tebbani S, Dumur D, Pareau D, Lopes F, Givry S, Entzmann F (2013). Modeling and parameter identification of the batch lactic acid production process from wheat flour. In proceedings of 17th International Conference on system Theory, Control and Computing—ICSTCC 2013, Romania. doi:10.1109/ICSTCC.2013.6688966
Gonzalez K, Tebbani S, Dumur D, Pareau D, Lopes F, Givry S, Entzmann F (2014) Control strategy for continuous lactic acid production from wheat flour. In: In Proceedings of 2nd International Conference on Control,Decision and Information Technologies—CoDIT’14. France, Metz. doi:10.1109/coDIT.2014.6996938
Hasanov H, Zakirova M, Boboev A, Akbarova N (2010) Enzymatic hydrolysis of various proteins of wheat in heterogeneous conditions. Int J BIOautomation 14:197–202
Herbert D, Elsworth R, Telling RC (1956) The continuous culture of bacteria: a theoretical and experimental study. J Gen Microbiol 14:601–622. doi:10.1099/00221287-14-3-601
Hetényi K, Németh A, Sevella B (2010) First steps in the development of a wheat flour-based lactic acid fermentation technology. Culture Medium Optimization Chem Biochem Eng Q 14:197–202
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative lactic acid production from renewable resources. Enzym Microb Tech 26:87–107. doi:10.1016/S0141-0229(99)00155-6
Huang LP, Jin B, Lant P, Zhou J (2005) Simultaneous saccharification and fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Biochem Eng J 23:265–276. doi:10.1016/j.bej.2005.01.009
Kwon S, Yoo IK, Lee WG, Chang HN, Chang YK (2001) High-rate continuous production of lactic acid by Lactobacillus rhamnosus in a two-stage membrane cell-recycle bioreactor. Biotechnol Bioeng 73:25–34. doi:10.1002/1097-0290(20010405)73:1<25::AID-BIT1034>3.0.CO;2-N
Levenspiel O (1980) The Monod equation: a revisit and generalization to product inhibition situation. Biotechnol Bioeng 22:1671–1687. doi:10.1002/bit.260220810
Luedeking R, Piret L (1959) A kinetic study of the lactic acid fermentation batch process at controlled pH. J Biochem Microbiol 1:393–412. doi:10.1002/jbmte.390010406
Pinelli D, Gonzalez-Vara A, Matteuzzi D, Magelli F (1997) Assessment of kinetic models for the production of L-and D-lactic acid isomers by Lactobacillus casei DMS 20011 and Lactobacillus coryniformis DMS 20004 in Continuous Fermentation. J Ferment Bioeng 83:209–212. doi:10.1016/S0922-338X(97)83586-6
Söndergård A, Stolt M (2002) Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci 27:1123–1163. doi:10.1016/S0079-6700(02)00012-6
Niemelä S, Keskus M (2002) Uncertainty of quantitative determinations derived by cultivation of microorganisms. Centre for Metrology and Accreditation, Finland
Trontel A, Bar V, Slavica A, Novak S (2010) Modelling the effect of different substrates and temperature on the growth and lactic acid production by Lactobacillus amylovorus DSM 20531T in batch process. Food Technol Biotechnol 48:352–361
Varadarajan S, Miller D (1999) Catalytic upgrading of fermentation-derived organic acids. Biotechnol Prog 156:845–854. doi:10.1021/bp9900965
Zhao B, Wang L, Li F, Hua D, Ma C, Ma Y, Xu P (2010) Kinetics of D-lactic acid production by Sporolactobacillus sp. strain CASD using repeated batch fermentation. Bioresour Technol 101:6499–6505. doi:10.1016/j.biortech.22010.03.069
Compliance with ethical standards
The authors declare that they have no competing interests.
This research does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
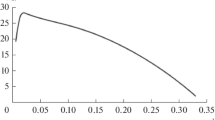
Appendix A: Values of variables at steady state as a function of the dilution rate
Appendix A: Values of variables at steady state as a function of the dilution rate
The lactic acid concentration at steady state from Eq. 7 can be represented by:
To determine the biomass concentration at steady state, the derivative in Eq. 2 was canceled, thus giving
where \( \overset{-}{X} \) is the biomass concentration at steady state. As \( \overline{\mu}=\overline{D} \), the biomass concentration at steady state can be represented as a function of the dilution rate at steady state introducing Eq. 7 in Eq. A.2 as follows:
The glucose concentration at steady state can be represented as a function of the biomass concentration by canceling the dynamics of Eq. 3
where \( \overset{-}{S} \) and \( \overset{-}{M} \) are the glucose and maltose concentrations at steady state, respectively. Equation A.4 shows that \( \overline{S} \) depends not only on the biomass concentration but also on the maltose concentration and on the dilution rate. From Eq. 4, it is possible to determine the maltose concentration at steady state:
Eq. A.5 defines the maltose concentration at steady state as a function of the dilution rate, replacing Eq. A.5 in Eq. A.4, it is possible to obtain:
From Eqs. A.6 and A.3, the glucose concentration at steady state becomes:
Eqs. A.1, A.3, A.5, and A.7 allow to express all variables at steady state only in terms of the dilution rate.
Rights and permissions
About this article
Cite this article
Gonzalez, K., Tebbani, S., Lopes, F. et al. Modeling the continuous lactic acid production process from wheat flour. Appl Microbiol Biotechnol 100, 147–159 (2016). https://doi.org/10.1007/s00253-015-6949-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6949-7