Abstract
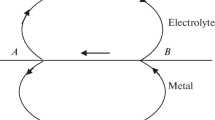
This work is a continuation of the studies of the nonstationary dependences (inclusion curves) recorded during the cathodic polarization of liquid electrodes made of lead, bismuth, cadmium, and aluminum in chloride melts, when the Marangoni-microeffect-induced structures in the form of vortices, i.e., circulation cells (CCs), dominate at the interface in the range from the current-free potential to the zero-charge potential. In this case, the time dependences of overvoltage η and current density i at steplike setting of the current under galvanostatic conditions or the potential under potentiostatic conditions (inclusion curves) have current or overvoltage extrema, which decrease until a steady state is formed. The values of η and i in the first extremum (ηext, iext) always have the highest amplitude. The time τext of reaching ηext and iext and the values of ηext and iext characterize the process of CC formation. τext is the most convenient for analysis, since it does not depend on the concentration of electrochemically active particles. We assume that the higher the mass-transfer processes at the electrode–electrolyte interface, which is determined by the polarization conditions, the slower the CC formation and the higher the value of τext. When the temperature increases, τext is shown to increase, which means that the CC formation proceeds more slowly. When an external magnetic field is applied to decelerate the mass transfer, τext decreases. When an electrode is immersed in a quartz holder, τext also decreases. These results generally indicate the correctness of the assumption about the factors influencing the rate of CC formation. When current pulses are sequentially applied to a polarized electrode, the inclusion curves contain a well-pronounced overvoltage extremum, the magnitude of which decreases in the pulse sequence. The values of τext also decrease. The existence of overvoltage extrema can be related to the energy difficulties of incorporating newly formed CCs into the structure of existing ones.





Similar content being viewed by others
REFERENCES
Yu. G. Mikhalev and N. Yu. Zharinova, “Nonstationary dependences during the polarization of liquid metal electrodes in molten salts with circulation cell structures,” Rasplavy, No. 4, 416–431 (2021).
Yu. G. Mikhalev, “Polarization and mass transfer during the electrolysis of molten salts with liquid metallic electrodes,” Russ. Metall. (Metally), No. 8, 599–605 (2014).
Yu. G. Mikhalev and L. A. Isaeva, “Structure in the form of a jet rising from the electrode–electrolyte interface,” Rasplavy, No. 3, 25–31 (2014).
Yu. G. Mikhalev and N. Yu. Zharinova, “Mass transfer conditions during the polarization of a liquid metal electrode in molten salts and the current efficiency,” Tsvetn. Met. 921 (9), 32–36 (2019).
T. Köllner, T. Boeck, and J. Schumacher, “Thermal Rayleigh–Marangoni convection in a three-layer liquid-metal-battery model,” Phys. Rev. E 95, 053114-1–053114-23 (2017).
H. Kim, D. A. Boysen, J. M. Newhouse, B. L. Spatocco, B. Chung, P. J. Burke, D. J. Bradwell, K. Jiang, A. A. Tomaszowska, K. Wang, et al., “Liquid metal batteries: past, present and future,” Chem. Rev. 113 (3), 2075–2099 (2012).
K. Wang, K. Jiang, B. Chung, T. Ouchi, P. J. Burke, D. A. Boysen, D. J. Bradwell, H. Kim, U. Muecke, and D. R. Sadoway, “Lithium–antimony–lead liquid metal battery for grid-level energy storage,” Nature (London) 514 (7522), 348–350 (2014).
V. P. Stepanov, Interphase Phenomena in Ionic Salt Melts (Nauka, Yekaterinburg, 1993).
Yu. G. Mikhalev, P. V. Polyakov, and S. P. Gerasimov, “Mass transfer at liquid metal electrodes during electrolysis of molten salts in a magnetic field (highly diluted melts),” Rasplavy, No. 2, 20–26 (1994).
Yu. G. Mikhalev and L. A. Isaeva, “Effect of geometric conditions on the mass-transfer parameters near a polarized liquid metallic electrode: I. Electrode size,” Russ. Metall. (Metally), No. 2, 153–158 (2014).
Yu. G. Mikhalev and N. Yu. Zharinova, “Effect of geometric conditions on the mass-transfer parameters near a polarized liquid metallic electrode. II. Shape and position of electrodes in a holder,” Rasplavy, No. 4, 333–350 (2020).
P. V. Polyakov, L. A. Isaeva, Yu. G. Mikhalev, and O. I. Bogdanovskii, “Mass transfer at a liquid electrode in the electrochemistry of molten salts,” Elektrokhimiya, No. 3, 302–307 (1979).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Mikhalev, Y.G., Zharinova, N.Y. Nonstationary Dependences during the Polarization of Liquid Metal Electrodes in Molten Salts with Circulation Cell Structures. 2. Influence of External Conditions and Sequentially Applied Current Pulses. Russ. Metall. 2022, 861–868 (2022). https://doi.org/10.1134/S0036029522080092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522080092