Abstract
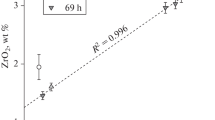
An isothermal saturation method was used to study the PbO solubility in the NaOH + 30 wt % Na2CO3 melt. A model dissolution mechanism is considered. Thermodynamic parameters have been calculated.
Similar content being viewed by others
References
O. G. Zarubitskii, Purification of Metals in Alkaline Melts (Metallurgiya, Moscow, 1981).
Yu. K. Delimarskii, P. P. Turov, and V. G. Gitman, “Recovery of lead from used batteries,” Ukr. Khim. Zh. 23 (6), 817–822 (1957).
A. G. Morachevskii, Z. I. Vaisgant, and A. I. Demidov, Processing of Secondary Lead Raw Materials (Khimiya, Sankt-Petersburg, 1993).
A. G. Morachevskii, Z. I. Vaisgant, and A. I. Demidov, Electrochemistry of Lead in Ionic Melts (Khimiya, St. Petersburg, 1994).
E. I. Speranskaya, “Interaction of lead oxide with sodium hydroxide,” Zh. Neorg. Khim. 6 (8), 1958–1959 (1961).
N. M. Barbin, V. F. Kazantsev, and N. A. Vatolin, Processing of Secondary Lead Raw Materials in Ionic Salt Melts (Izd. UrO RAN, Yekaterinburg, 2002).
G. Brauer, Handbuch der Praparativen Anorganischen (Ferdinand Enke, Stuttgart 1975), Vol. 3.
Yu. V. Karyakin and I. I. Angelov, Pure Chemical Reagents (Goskhimizdat, Moscow, 1955).
N. M. Barbin, V. N. Nekrasov, and L. E. Ivanovskii, “Dilithium oxide solubility in equimolar NaCl–KCl molten mixture,” Rasplavy, No. 2, 117–120 (1990).
N. M. Barbin, A. P. Pekar’, and V. N. Nekrasov, “Solubility of alkaline-earth metal oxides in equimolar NaCl–KCl molten mixture,” Rasplavy, No. 2, 41–48 (1992).
R. Ripan and I. Chetyanu, Inorganic Chemistry (Mir, Moscow, 1971), Vol. 1.
Thermodynamic Properties of Individual Substances, Ed. by V. P. Glushko (Nauka, Moscow, 1981), Vol. 3.
L. I. Antropov and D. A. Tkalenko, “Oxygen reduction in alkaline melts. II. Thermodynamics of oxygen–alkaline melt system,” Elektrokhimiya 6 (6) 1557–1560 (1970).
G. Doisheau and B. Tremillon, “Proprietes chimigues et electrihimigues dans les hydroxydes alcalins fondus,” J. Chim. Phys. 71 (11–12), 1445–1554 (1974).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.M. Barbin, E.G. Viskova, O.V. Pashchenko, 2016, published in Rasplavy, 2016, No. 4, pp. 286–290.
Rights and permissions
About this article
Cite this article
Barbin, N.M., Viskova, E.G. & Pashchenko, O.V. Thermodynamic analysis of the lead oxide dissolution in the alkali–carbonate NaOH + 30 wt % Na2CO3 melt. Russ. Metall. 2017, 79–81 (2017). https://doi.org/10.1134/S0036029517020033
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029517020033