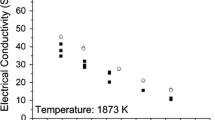
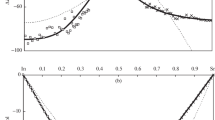
Abstract
The relation between the activation energy and the preexponential factor in the Arrhenius equation that describes the viscosity and electrical conductivity of oxide (slag) melts is systematically analyzed over wide composition and temperature ranges for the first time. When deriving this relation, we do not use any model concepts and assumptions concerning the structure of slag melts or the character of electric charge transfer in them. The fundamental applicability of the Meyer-Neldel rule for this relation is shown and grounded. The application of this algorithm in practice can give information on the structure of experimental data, nonobvious correlations, and possible relations of a higher order and can quantitatively predict the behavior of parameters, including the range outside experimentally determined data.
Similar content being viewed by others
References
S. Srldhar, “Estimation models for molten slag and alloy viscosities,” J. Metals 11, 46–50 (2002).
A. Kondratiev, E. Jak, and P. C. Hayes, “Predicting slag viscosities in metallurgical systems,” J. Metals 11, 41–45 (2002).
T. Iida, Z. Morita, and T. Mizobuchi, “Relationships between a parameter deduced from viscosity and some physico-chemical properties of molten slags,” in Proceedings of 3rd International Conference on Molten Slags and Fluxes (Institute of Metals, London, 1988), pp. 199–202.
S. Seetharaman and D. Sichen, “Estimation of the viscosities of binary metallic melts using Gibbs energies of mixing,” Met. Mater. Trans. B 25, 589–595 (1994).
L. Segers, A. Fontana, and R. Winand, “Conductivities electriques de melanges de silicates fondus du systeme CaO-SiO2-MnO,” Electroch. Acta 23, 1281–1286 (1978).
M. I. Gasik and V. A. Gavrilov, Silicothermy of Manganese (Sistemnye Tekhnologii, Dnepropetrovsk, 2001).
M. I. Gasik, Manganese (Metallurgiya, Moscow, 1992).
V. Ya. Dashevskii, I. A. Karyazin, and V. I. Kashin, “Viscosity of oxide melts based on manganese oxide,” Izv. Ross. Akad. Nauk, Ser. Met., No. 1, 28–32 (1984).
V. N. Kornoukhov, Yu. I. Voronov, V. P. Zaiko, and V. I. Zhuchkov, Technology of Low-Carbon Ferrochrome (Izd. UrO RAN, Yekaterinburg, 2001).
Yu. I. Voronov, V. P. Zaiko, and V. I. Zhuchkov, Technology of Molybdenum-Containing Ferroalloys (Izd. UrO RAN, Yekaterinburg, 2000).
F. Shahbazian, “Experimental studies of the viscosities in the CaO-FeO-SiO2-CaF2 slags,” Scand. J. Metall. 30, 302–308 (2001).
M. M. Gasik and M. I. Gasik, “Multi-variation analysis and optimisation of electrical conductivity of MnO-CaO-SiO2 slags,” in Proceedings of 12th International Ferroalloys Congress, Ed. by A. Vartiainen (Helsinki, 2010), Vol. 2, pp. 537–545.
W. Meyer and H. Neldel, “Über die beziehungen zwischen der energiekonstanten e under der mengenkonstanten a in der leitwerts-temperaturformel bei oxydischen halbleitern,” Z. Tech. Physik B 18, 588–593 (1937).
R. Metselaar and G. Oversluizen, “The Meyer-Neldel rule in semiconductors,” J. Solid State Chem. 55, 320–326 (1984).
J. W. Niemantsverdriet, K. Markert, and K. Wandelt, “The compensation effect and the manifestation of lateral interactions in thermal desorption spectroscopy,” Appl. Surf. Sci. 31, 211–219 (1988).
W. Bogusz, D. E. Kony, and F. Krok, “Application of the Meyer-Neldel rule to the electrical conductivity of Nasicon,” Mater. Sci. Eng. B: Solid-State Mater. Adv. Technol. 15, 169–172 (1992).
G. C. Bond, “Kinetics of alkane reactions on metal catalysts: activation energies and the compensation effect,” Catal. Today 49, 41–47 (1999).
R. Arora and A. Kumar, “Electrical conduction in chalcogenide glasses. Applicability of the Meyer-Neldel rule,” Phys. Stat. Sol. A 1125, 273–278 (1991).
S. K. Dwivedi, M. Dixit, and A. Kumar, “Pre-exponential factor in semiconducting chalcogenide glasses,” J. Mater. Sci. Lett. 17, 233–235 (1998).
N. Mehta, K. Singh, and N. S. Saxena, “Correlation between pre-exponential factor and activation energy of non-isothermal crystallization for virgin and irradiated Fe78B13Si9 metallic glass,” Physica B 404, 2184–2188 (2009).
R. A. Swalin, “Correlation between frequency factor and activation energy for solute diffusion,” J. Appl. Phys. 27, 554–555 (1956).
D. Lazarus, “Effect of screening on solute diffusion in metals,” Phys. Rev. 93, 973–976 (1954).
J. C. Dyre, “A phenomenological model for the Meyer-Neldel rule,” J. Phys. C: Solid State Phys. 19, 5655–5664 (1986).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.M. Gasik, M.I. Gasik, L.I. Leont’ev, V.Ya. Dashevskii, K.V. Griogorovich, 2014, published in Metally, 2014, No. 4, pp. 3–9.
Rights and permissions
About this article
Cite this article
Gasik, M.M., Gasik, M.I., Leont’ev, L.I. et al. Fundamental relation between the main parameters of the thermally activated transport phenomena in complex oxide melts. Russ. Metall. 2014, 503–508 (2014). https://doi.org/10.1134/S0036029514070052
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029514070052