Abstract
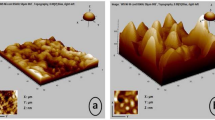
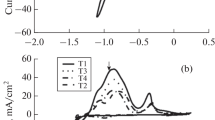
To enhance the corrosion resistance of pure aluminum in sulfur-containing environments, Ni–Mo coatings were deposited on aluminum substrates via direct current (DC) and pulse current (PC) electrodeposition techniques. The phase structure, micromorphology and element composition of the coatings were characterized by XRD, SEM, and EDS. The corrosion resistance of pure aluminum and Ni–Mo coatings in 1 wt % Na2S aqueous solution were studied by static corrosion immersion and electrochemical test. The results indicated that aluminum suffered severe corrosion in Na2S aqueous solution, leading to the formation of Al(OH)3. The application of Ni–Mo coatings effectively prevented direct contact between the solution and the aluminum substrate. Compared with the coating prepared by DC electrodeposition under the same conditions, the Ni–Mo coating obtained through PC electrodeposition exhibited finer grains, a more compact and flatter surface, and higher Mo content, the impedance value was 15.61 kΩ cm2 and its corrosion current density was 9.610 × 10–6 A/cm2. The corrosion current density of the coating prepared by DC electrodeposition was 1.514 × 10–5 A/cm2, whereas that of pure aluminum in the same medium was 3.030 × 10–3 A/cm2.








Similar content being viewed by others
REFERENCES
O. Ur Rehman and Y. Ali, Transp. Res. E Logist. Transp. Rev. 148, 102246 (2021).
M. Yuan, H. R. Zhang, B. H. Wang, et al., Energy Policy 136, 111077 (2020).
Z. M. Wang and J. Zhang, 34, 17 (2016).
H. H. Jasim, Mater. Corros. 67, 988 (2016).
Gamal, A. M. Hossam, Zewail, et al., Corros. Rev. 36, 483 (2018).
Y. B. Peng, G. Wen, X. L. Gou, et al., Sep. Purif. Technol. 202, 111 (2018).
Y. Lv, M. Y. Liu, and Y. S. H. Xu, Prot. Met. Phys. Chem. Surf. 54, 526 (2018).
M. El Kamel, A. Galtayries, Ph. Vermaut, et al., Surf. Interface Anal. 42, 605 (2010).
T. Stephenson, M. Hazelton, M. Kupsta, et al., Fuel 139, 411 (2015).
R. R. Yang, Z. R. Zhang, J. C. Jiang, et al., Eng. Failure Anal. 108, 104342 (2020).
G. R. Osorio-Celestino, M. Hernandez, D. Solis-Ibarra, et al., ACS Omega 5, 17304 (2020).
E. Z. Hu, Y. F. Xu, X. G. Hu, et al., Renewable Energy 37, 371 (2012).
Y. C. Wang, L. F. Cao, X. D. Wu, et al., Mater. Rev. 33, 1190 (2019).
M. Xiang, Y. Yuan, X. He, et al., High Volt. Eng. 45, 484 (2019).
X. Y. Cao, Y. W. Lu, W. R. Wang, et al., Chem. Eng. Commun. 210, 233 (2022).
R. R. Yang, Z. R. Wang, J. C. Jiang, et al., Eng. Failure Anal. 108, 104342 (2020).
T. Ando and Y. Harada, Zairyo-to-Kankyo 54, 201 (2005).
Y. Y. Liu, J. M. Huang, J. B. Claypool, et al., Appl. Surf. Sci. 355, 805 (2015).
Q. Yu, S. X. Yu, Q. Xing, et al., J. Mater. Eng. Perform. 28, 5725 (2019).
A. Tanji, F. Gapsari, A. Syahrom, et al., J. Alloys Compd. 871, 159582 (2021).
Y. L. Chou, J. W. Yeh, and H. C. Shih, Corros. Sci. 52, 2571 (2010).
A. Elbiache and P. Marcus, Corros. Sci. 33, 261 (1992).
V. Rajaei, H. Rashtchi, K. Raeissi, et al., Int. J. Hydrogen Energy 42, 14264 (2017).
A. Laszczyńska, W. Tylus, J. Winiarski, et al., Surf. Coat. Technol. 317, 26 (2017).
Y. K. Xu, S. M and M. Y. Fan, et al., Surf. Coat. Technol. 363, 51 (2019).
R. Y. Zhang, Z. L. Li and X. Yu, et al., Surf. Eng. 35, 578 (2019).
Kung-Hsu Hou and Yann-Cheng Chen, Appl. Surf. Sci. 257, 6340 (2011).
H. Goldasteh and S. Rastegari, Surf. Coat. Technol. 259, 393 (2014).
S. Lakra, H. S. Maharana, and A. Basu, Mater. Manuf. Proces. 31, 1447 (2016).
P.-Ch. Huang, K.-H. Hou, G.-L. Wang, et al., Int. J. Electrochem. Sci. 10, 4972 (2015).
Q. L. Bao, W. X. Zheng, L. Chen, et al., Colloids Surf., A 636, 128128 (2022).
R. Mousavi, M. Esmailzadeh, and F. Deflorian, Mater. Res. Express 6, 056534 (2019).
S. K. Singh, S. Samanta, A. K. Das, et al., Surf. Topogr.: Metrol. Prop. 7 (3), 1 (2019).
J. J. Gray, B. S. El Dasher, and C. A. Orme, Surf. Sci. 600, 2488 (2006).
C. Liu, X. Huang, R. Xu, et al., J. Mater. Eng. Perform. 30, 2514 (2021).
Funding
This work was supported by the National Natural Science Foundation of China no. 1 under grant no. 21203095; the Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM) no. 2; and the Priority Academic Program Development of Jiangsu Higher Education Institutions no. 3.
Author information
Authors and Affiliations
Contributions
Xu Li: conceptualization, writing—review and editing, methodology. Zengzeng Zheng: investigation, writing—original draft. Xujie Xiao: data curation, resources. Jingkang Chen: validation visualization, software. Chengfei Zhu: writing—review and editing, funding acquisition.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, X., Zheng, Z., Xiao, X. et al. Corrosion Behavior of Ni–Mo Coatings Prepared by Different Electrodeposition Methods in Na2S Solution. Russ. J. Phys. Chem. 97, 2846–2854 (2023). https://doi.org/10.1134/S0036024423120300
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423120300