Abstract
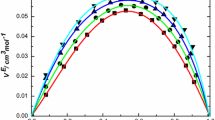
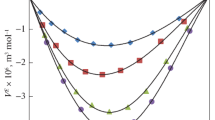
In the present study, the new experimental values of thermophysical properties namely density (ρ) and viscosity (η) for binary liquid mixtures of benzyl alcohol (BA) with n-butylamine (n-BA), sec-butylamine (s-BA) and tert-butylamine (t-BA) have been measured over the whole range of mole fraction of benzyl alcohol at atmospheric pressure and at 298.15, 303.15, and 308.15 K temperatures. Using the thermophysical data, excess molar volume (\(V_{m}^{E}\)), viscosity deviation (∆η), and also apparent molar volumes (\({{V}_{{m,\varnothing ,1}}}~\) and \({{V}_{{m,\varnothing ,2}}}\)) were calculated. Further, the Redlich–Kister (R–K) equation was used to correlate \(V_{m}^{E}\) and ∆η properties at all worked temperatures. Based on obtained results intermolecular interactions (H-bond and \({{\pi }}{-} {\text{HN}}\)) were determined. The \(V_{m}^{E}\) values were found to be negative deviations and ∆η values were found to be positive deviations over the full range of mole fraction of benzyl alcohol for all systems. Furthermore, the effect of temperature on ρ, η, \(V_{m}^{E}\), and ∆η was also informed.










Similar content being viewed by others
REFERENCES
G. Douhéret and M. I. Davis, Chem. Soc. Rev. 22, 43 (1993).
M. J. Blandamer, M. I. Davis, G. Douheret, and J. C. Reis, Chem. Soc. Rev. 30, 8 (2001).
P. Venkatesu, Fluid Phase Equilib. 298, 173 (2010).
B. Satheesh, D. Sreenu, M. Chandrasekhar, and T. S. Jyostna, J. Mol. Liq. 317, 113942 (2020).
S. L. Oswal and M. M. Maisuria, J. Mol. Liq. 100, 91 (2002).
S. L. Oswal and M. M. Maisuria, J. Mol. Liq. 100, 91 (2002).
G. Nath, S. Sahu, and R. Paikaray, Ind. J. Phys. 83, 429 (2009).
G. Conti, P. Gianni, L. Lepori, and E. Matteoli, J. Pure Appl. Chem. 67, 1849 (1995).
S. Singh, B. P. Sethi, R. C. Katyal, and V. K. Rattan, J. Chem. Eng. Data 49, 1373 (2004).
B. González, N. Calvar, E. Gómez, and A. Domínguez, J. Chem. Thermodyn. 39, 1578 (2007).
S. C. Bhatia, J. Sangwan, and R. Bhatia, J. Mol. Liq. 161, 95 (2001).
I. C. Hwang, K. L. Kim, and S. J. Park, J. Chem. Eng. Data 52, 1919 (2007).
X. P. Wang, F. X. Yang, and Y. Gao, J. Chem. Thermodyn. 57, 145 (2013).
R. S. Neyband, A. Yousefi, and H. Zarei, J. Chem. Eng. Data 60, 2291 (2015).
The Merck Index, 13th ed. (Merck & Co., Whitehouse Station, NJ, 2001).
K. B. Ranjith, P. M. Krishna, S. A. Banu, K. A. Jyothi, T. S. Savitha, and N. Satyanarayana, Phys. Chem. Liq. 48, 79 (2010).
Ullmann's Encyclopedia of Industrial Chemistry, 5th ed. (VCH, Weinheim, 1985), Vol. A2, p. 9.
G. S. Reddy, A. S. Reddy, M. V. Subbaiah, and A. Krishnaiah, J. Sol. Chem. 39, 399 (2010).
W. L. Weng and J. T. Chen, J.Chem. Eng. Data 49, 1748 (2004).
T. S. Jyostna, B. Satheesh, D. Sreenu, G. Ramesh, and R. E. Jayanthi, Phys. Chem. Liq. 58, 349 (2020).
S. Sharma and M. Makavana, Fluid Phase Equilib. 375, 219 (2014).
M. Moosavi and A. A. Rostami, J. Chem. Eng. Data 62, 156 (2017).
S. Azizian and N. Bashavard, J. Chem. Eng. Data 50, 1303 (2005).
S. N. Pandharinath and S. J. Kharat, J. Chem. Eng. Data 48, 1291 (2003).
A. Pal, R. Gaba, and S. Sharma, J. Chem. Eng. Data 53, 1643 (2008).
C. M. Kinart, W. J. Kinart, D. Chęcińska-Majak, and A. Ćwiklińska, J. Mol. Liq. 109, 19 (2004).
S. L. Oswal, P. Oswal, and R. L. Gardas, Fluid Phase Equilib. 216, 33 (2004).
F. L. Chowdhury and M. A. Saleh, J. Mol. Liq. 191, 156 (2014).
C. S. M. Subha, S. G. Narayana, and B. M. Eshwari, Ind. J. Chem. 43A, 1876 (2000).
M. A. Saleh, S. Akhtar, and A. R. Khan, Phys. Chem. Liq. 39, 85 (2001).
C. Hao, Z. Zhao, X. Yue, Y. Pang, and J. Zhang, J. Mol. Liq. 274, 730 (2019).
H. Shi, L. Ma, B. Zhao, Y. Pang, and Z. Wu, J. Mol. Liq. 250, 182 (2018).
M. Raveendra, M. Chandrasekhar, C. Narasimharao, C. Venkatramanna, K. S. Kumar, and K. D. Reddy, RSC Adv. 6, 27335 (2016).
T. H. Nam, J. Phys. Chem. 98, 5362 (1994).
G. Larsen, Z. K. Ismail, B. Herreros, and R. D. Parra, J. Phys. Chem. A 102, 4734 (1998).
I. Bahadur and N. Deenadayalu, J. Sol. Chem. 40, 1528 (2011).
M. V. Rathnam, R. T. Sayed, K. R. Bhanushali, and M. S. S. Kumar, J. Mol. Liq. 166, 9 (2012).
K. Rajagopal and S. Chenthinath, J. Mol. Liq. 155, 20 (2010).
S. Parveen, S. Singh, D. Shukla, M. Yasmin, M. Gupta, and J. P. Shukla, J. Sol. Chem. 41, 156 (2012).
ACKNOWLEDGMENTS
The author Sumalatha Donthula thankful to Chaitanya Deemed to be University, Hanamkonda for the constant support and encouragement during this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Donthula, S., Raju, A. Thermophysical Studies on Binary Liquid Mixtures of Benzyl Alcohol and Alkylamines at 298.15–308.15 K. Russ. J. Phys. Chem. 97, 1849–1859 (2023). https://doi.org/10.1134/S0036024423090236
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423090236