Abstract
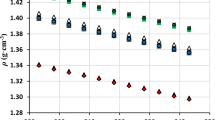
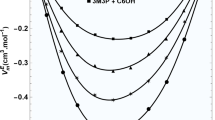
Densities, speeds of sound, and viscosities of binary mixtures of methyl benzoate (MB) with ethanol, 1-propanol, and 1-butanol were measured at temperature ranging from T = 293.2 to 313.2 K and refractive indices were determined at T = 303.2 K. Excess molar volumes, VE, isentropic compressibility deviations ∆κs and viscosity deviations, Δη, were obtained and fitted with the Redlich–Kister equation with satisfactory results. Based on the measured and derived physicochemical properties, the influence of the alkyl chain length of the alcohol on the molecular interactions of the studied mixtures was explored. The results show that both hydrogen bond interaction between carbonyl group of MB and hydroxyl group of alcohol and the packing efficiency, such as accommodation of alcohol in the interstice of MB, play an important role in the microstructure of the mixtures, which leads to the disruption of alcohol with the addition of MB. Moreover, by comparing VE, ∆κs and Δη values of MB (1) + alcohol (2) systems at T = 303.2 K, it is found that the strength of hydrogen bond interaction and the packing efficiency significantly weakens with increasing alkyl chain length of alcohol in going from ethanol to 1-butanol, resulting in the VE values of the studied systems gradually changing from a negative deviation to an S shape, the ∆κs and Δη values have negative deviations, and the ∆nD values have positive deviations. The obtained results will provide thermodynamic guidance for the industrial purification of methyl benzoate.




Similar content being viewed by others
References
Jyothirmai, G., Nayeem, S.M., Khan, I., Anjaneyulu, C.J.: Thermo-physicochemical investigation of molecular interactions in binary combination (dimethyl carbonate + methyl benzoate). Therm. Anal. Calorim. 132, 693–707 (2018)
Werkmeister, S., Junge, K., Wendt, B., Alberico, E., Jiao, H., Baumann, W., Junge, H., Gallou, F., Beller, M.: Hydrogenation of esters to alcohols with a well-defined iron complex. Angew. Chem. Int. Ed. 53, 8722–8726 (2014)
Jiang, Y., Bao, Q., Gui, W., Wu, Y., Liu, X., Zhang, L., Zheng, B., Wang, Z.: A highly effective Cu/ZnO/Al2O3 catalyst for hydrogenation of methyl benzoate to benzyl alcohol in methanol solution. Catal. Lett. 149, 1359–1367 (2019)
Li, Y., Liu, Q., Huang, W., Yang, J.: Solubilities of CO2 capture absorbents methyl benzoate, ethyl hexanoate and methyl heptanoate. J. Chem. Thermodyn. 127, 25–32 (2018)
Ardizzone, S., Bianchi, C.L., Ragaini, V., Vercelli, B.: SO4–ZrO2 catalysts for the esterification of benzoic acid to methylbenzoate. Catal. Lett. 62, 59–65 (1999)
Kamitanaka, T., Yamamoto, K., Matsuda, T., Harada, T.: Transformation of benzonitrile into benzyl alcohol and benzoate esters in supercritical alcohols. Tetrahedron 64, 5699–5702 (2008)
Heintz, A.: Recent developments in thermodynamics and thermophysics of non-aqueous mixtures containing ionic liquids. A review. J. Chem. Thermodyn. 37, 525–535 (2005)
Douhéret, G., Davis, M.I., Reis, J.C.R., Blandamer, M.J.: Isentropic compressibilities—experimental origin and the quest for their rigorous estimation in thermodynamically ideal liquid mixtures. Chem. Phys. Chem. 2, 148–161 (2001)
Gmehling, J., Kolbe, B., Kleiber, M., Rarey, J.: Chemical Thermodynamics for Process Simulation. Wiley–VCH (2019)
Sastry, S.S., Shaik, B., Vishwam, T., Ha, S.T.: Excess thermodynamic and acoustic properties for the binary mixtures of methyl benzoate at T = (303, 308, 313, 318 and 323) K. Phys. Chem. Liq. 52, 272–286 (2014)
Sinnokrot, M.O., Valeev, E.F., Sherrill, C.D.: Estimates of the ab initio limit for π-π interactions: the benzene dimer. J. Am. Chem. Soc. 124, 10887–10893 (2002)
Navarro, A.M., García, B., Hoyuelos, F.J., Peñacoba, I.A., Leal, J.M.: preferential solvation in alkan-1-ol/alkylbenzoate binary mixtures by solvatochromic probes. J. Phys. Chem. B 115, 10259–10269 (2011)
Umadevi, M., Kesavasamy, R., Rathina, K., Mahalakshmi, R.: Studies on liquid–liquid interactions of some ternary mixtures by density, viscosity and ultrasonic speed measurements. J. Mol. Liq. 219, 820–828 (2016)
Shihab, S.S., Rao, K.G., Kiran, M.G., Babu, S., Sastry, S.S.: Excess thermodynamic and acoustic properties for equimolar mixture of methyl benzoate and alkanols with benzene at 303.15 K. Rasayan. J. Chem. 10, 59–63 (2017)
Rao, M.V.P., Naidu, P.R.: Excess volumes of binary mixtures of alcohols in methylcyclohexane. Can. J. Chem. 52, 788–790 (1974)
Garcia, B., Ortega, J.C.: Excess viscosity ηE, excess volume VE, and excess free energy of activation ∆G*E at 283, 293, 303, 313, and 323 K for mixtures acetonitrile and alkyl benzoates. J. Chem. Eng. Data 33, 200–204 (1988)
Aminabhavi, T.M., Phayde, H.T., Khinnavar, R.S., Gopalakrishna, B., Hansen, K.C.: Densities, refractive indices, speeds of sound, and shear viscosities of diethylene glycol dimethyl ether with ethyl acetate, methyl benzoate, ethyl benzoate, and diethyl succinate in the temperature range from 298.15 to 318.15 K. J. Chem. Eng. Data 39, 251–260 (1994)
Rathnam, M.V., Sayed, R.T., Bhanushali, K.R., Kumar, M.S.S.: Molecular interaction study of binary mixtures of methyl benzoate: viscometric and ultrasonic study. J. Mol. Liq. 166, 9–16 (2012)
Muñoz, M.M., Tinjacá, D.A., Jouyban, A., Martínez, F., Acree, W.E.: Study of some volumetric and refractive properties of PEG 300 (1) + ethanol (2) mixtures at several temperatures. Phys. Chem. Liq. 56, 391–402 (2018)
Chen, J., Chen, L., Xu, Y.: Properties of pure 1,1,3,3-tetramethylguanidine imidazole ionic liquid and its binary mixtures with alcohols at T = (293.15 to 313.15) K. J. Chem. Thermodyn. 88, 110–120 (2015)
Dzida, M., Żak, A., Ernst, S.: Thermodynamic and acoustic properties of binary mixtures of alcohols and alkanes. I. Speed of sound in (ethanol + n-heptane) under elevated pressures. J. Chem. Thermodyn. 37, 405–414 (2005)
Li, J., Hu, Y., Sun, S., Liu, Y., Liu, Z.: Densities and dynamic viscosities of the binary system (water + 1-hexyl-3-methylimidazolium bromide) at different temperatures. J. Chem. Thermodyn. 42, 904–908 (2010)
Trenzado, J., Romano, E., Segade, L., Caro, M.N., González, E., Galván, S.: Densities and viscosities of four binary diethyl carbonate + 1-alcohol systems from (288.15 to 313.15) K. J. Chem. Eng. Data 56, 2841–2848 (2011)
Sadeghi, R., Azizpour, S.: Volumetric, compressibility, and viscometric measurements of binary mixtures of poly (vinylpyrrolidone) + water, + methanol, + ethanol, + acetonitrile, + 1-propanol, + 2-propanol, and + 1-butanol. J. Chem. Eng. Data 56, 240–250 (2011)
George, J., Sastry, N.V.: Densities, dynamic viscosities, speeds of sound, and relative permittivities for water + alkanediols (propane-1, 2-and-1, 3-diol and butane-1, 2-,-1, 3-,-1, 4-, and-2, 3-diol) at different temperatures. J. Chem. Eng. Data 48, 1529–1539 (2003)
Rodriguez, A., Canosa, J., Tojo, J.: Density, refractive index, and speed of sound of binary mixtures (diethyl carbonate + alcohols) at several temperatures. J. Chem. Eng. Data 46, 1506–1515 (2001)
Dubey, G.P., Sharma, M., Dubey, N.: Study of densities, viscosities, and speeds of sound of binary liquid mixtures of butan-1-ol with n-alkanes (C6, C8, and C10) at T = (298.15, 303.15, and 308.15) K. J. Chem. Thermodyn. 40, 309–320 (2008)
Nain, A.K.: Volumetric, acoustic and viscometric studies of solute-solute and solute-solvent interactions of isoniazid in aqueous-glucose/sucrose solutions at temperatures from 293.15 K to 318.15 K. J. Chem. Thermodyn. 133, 123–134 (2019)
Stokes, R.H., Mills, R.: Viscosity of Electrolytes and Related Properties. Pergamon Press, New York (1965)
Reddy, M.S., Raju, K.T.S., Rao, A.S., Sharmila, N., Babu, B.H.: Study of thermophysical properties of the binary mixtures of ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate and 2-propoxyethanol from T = (298.15 To 328.15) K at atmospheric pressure. J. Chem. Thermodyn. 101, 139–149 (2016)
Wang, J., Tian, Y., Zhao, Y., Zhuo, K.: A volumetric and viscosity study for the mixtures of 1-n-butyl-3-methylimidazolium tetrafluoroborate ionic liquid with acetonitrile, dichloromethane, 2-butanone and n, n–dimethylformamide. Green Chem. 5, 618–622 (2003)
Mokhtarani, B., Sharifi, A., Mortaheb, H.R., Mirzaei, M., Mafi, M., Sadeghian, F.: Densities and viscosities of pure 1-methyl-3-octylimidazolium nitrate and its binary mixtures with alcohols at several temperatures. J. Chem. Eng. Data 55, 3901–3908 (2010)
Xu, Y., Yao, J., Wang, C., Li, H.: Density, viscosity, and refractive index properties for the binary mixtures of n-butylammonium acetate ionic liquid + alkanols at several temperatures. J. Chem. Eng. Data 57, 298–308 (2012)
Abria, A., Babajani, N., Zonouz, A.M., Shekaari, H.: Spectral and thermophysical properties of some novel deep eutectic solvent based on l-menthol and their mixtures with ethanol. J. Mol. Liq. 285, 477–487 (2019)
Grimme, S.: Do special noncovalent π–π stacking interactions really exist? Angew. Chem. Int. Ed. 47, 3430–3434 (2008)
Zhu, A., Wang, J., Liu, R.: A volumetric and viscosity study for the binary mixtures of 1-hexyl-3-methylimidazolium tetrafluoroborate with some molecular solvents. J. Chem. Thermodyn. 43, 796–799 (2011)
Feng, X., Chen, T., Yin, Y., Xu, Y.: A detail study of the microstructure of methyl benzoate/methanol mixture proved by IR spectra, excess infrared wavenumber, and physicochemical properties. J. Mol. Liq. 302, 112521 (2020)
Brooks, N.J., Castiglione, F., Doherty, C.M., Dolan, A., Hill, A.J., Hunt, P.A., Matthews, R.P., Mauri, M., Mele, A., Simonutti, R., Villar-Garcia, I.J., Weber, C.C., Welton, T.: Linking the structures, free volumes, and properties of ionic liquid mixtures. Chem. Sci. 8, 6359–6374 (2017)
Pires, J., Timperman, L., Jacquemin, J., Balducci, A., Anouti, M.: Density, conductivity, viscosity, and excess properties of (pyrrolidinium nitrate-based protic ionic liquid + propylene carbonate) binary mixture. J. Chem. Thermodyn. 59, 10–19 (2013)
Anouti, M., Vigeant, A., Jacquemin, J., Brigouleix, C., Lemordant, D.: Volumetric properties, viscosity and refractive index of the protic ionic liquid, pyrrolidinium octanoate, in molecular solvents. J. Chem. Thermodyn. 42, 834–845 (2010)
Xu, Y., Chen, B., Qian, W., Li, H.: Properties of pure n-butylammonium nitrate ionic liquid and its binary mixtures of with alcohols at T = (293.15 to 313.15) K. J. Chem. Thermodyn. 58, 449–459 (2013)
Govinda, V., Venkatesu, P., Bahadur, I.: Molecular interactions between ammonium-based ionic liquids and molecular solvents: current progress and challenges. Phys. Chem. Chem. Phys. 18, 8278–8326 (2016)
Aggarwal, N., Sharma, M., Banipal, T.S., Banipal, P.K.: Influence of phosphate-based salts on enthalpy of dilution and isentropic compressibility properties of saccharides and their derivatives in aqueous solutions. J. Chem. Eng. Data 64, 517–528 (2019)
Orge, B., Rodríguez, A., Canosa, J.M., Marino, G., Iglesias, M., Tojo, J.: Variation of densities, refractive indices, and speeds of sound with temperature of methanol or ethanol with hexane, heptane, and octane. J. Chem. Eng. Data 44, 1041–1047 (1999)
Umapathi, R., Naidoo, P., Ramjugernath, D., Venkatesu, P., Bahadur, I.: Investigation of temperature and composition dependence of molecular interactions between phosphonium-based ionic liquid + N, N-dimethylformamide: A study of thermophysical properties. J. Mol. Liq. 291, 110987 (2019)
Rana, V.A., Chaube, H., Gadani, D.H.: Dielectric permittivity, density, viscosity and refractive index of binary mixtures of anisole with methanol and 1-propanol at different temperatures. J. Mol. Liq. 164, 191–196 (2011)
Rana, V.A., Chaube, H.A.: Static permittivity, density, viscosity and refractive index of binary mixtures of anisole with 1-butanol and 1-heptanol at different temperatures. J. Mol. Liq. 173, 71–76 (2012)
Fort, J.R., Moore, W.R.: Viscosities of binary liquid mixtures. Trans. Faraday Soc. 62, 1112–1119 (1966)
Anouti, M., Jacquemin, J., Lemordant, D.: Volumetric properties, viscosities, and isobaric heat capacities of imidazolium octanoate protic ionic liquid in molecular solvents. J. Chem. Eng. Data 55, 5719–5728 (2010)
An, N., Zhuang, B., Li, M., Lu, Y., Wang, Z.G.: Combined theoretical and experimental study of refractive indices of water–acetonitrile–salt systems. J. Phys. Chem. B 119, 10701–10709 (2015)
Deetlefs, M., Seddon, K.R., Shara, M.: neoteric optical media for refractive index determination of gems and minerals. New J. Chem. 30, 317–326 (2006)
Díaz-Rodríguez, P., Cancilla, J.C., Plechkova, N.V., Matute, G., Seddon, K.R., Torrecilla, J.S.: Estimation of the refractive indices of imidazolium-based ionic liquids using their polarisability values. Phys. Chem. Chem. Phys. 16, 128–134 (2014)
Zhu, J., Xu, Y., Feng, X., Zhu, X.: A Detailed study of physicochemical properties and microstructure of EmimCl-EG deep eutectic solvents: Their influence on SO2 absorption behavior. J. Ind. Eng. Chem. 67, 148–155 (2018)
Ye, F., Zhu, J., Yu, K., Zhu, R., Xu, Y., Chen, J., Chen, L.: physicochemical properties of binary mixtures of 1,1,3,3-tetramethylguanidine imidazolide ionic liquid with water and alcohols. J. Chem. Thermodyn. 97, 39–47 (2016)
Acknowledgements
This work was supported by the National Science Foundation of China (No. 21978172), National Natural Science Foundation of Zhejiang Province (No. Y18B060014), National Undergraduate Training Program for Innovation and Entrepreneurship (No. 201810349013), Undergraduate Scientific and Technological Innovation Project of Shaoxing University, the Key Teacher Training Research of Shaoxing University (No. 2016227).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, T., Feng, X., Yin, Y. et al. Investigation of the Effect of Alkyl Chain Length on Molecular Interactions Between Methyl Benzoate with Alcohols: A Study of Physicochemical Properties. J Solution Chem 49, 1402–1418 (2020). https://doi.org/10.1007/s10953-020-01029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01029-4