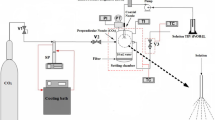
Abstract
A possible mechanism is considered for the formation of nanoscale oxides based on titanium and aluminum isopropoxides in a medium of supercritical CO2 fluid. It is shown that because of intermolecular interactions and high pressure in the system, the supercritical fluid acquires the properties of a condensed medium, the main role of which is to restrain processes of hydrolysis. At the first stage of the hydrolysis of titanium isopropoxide, the water molecule is coordinated in the outer sphere of the central atom due to the formation of intermolecular hydrogen bonds. It is then coordinated into the inner sphere with the formation of a five-coordinate transition state and its destruction, creating a product substituted for the hydroxo group. The next steps proceed in a similar way. The described mechanism agrees with experimental findings and produces nanosized X-ray amorphous titanium oxide. (With aluminum isopropoxide, only the hydrolyzed hydroxo form can be produced.) Results suggest the production of nanosized oxides from isopropoxides in a medium of supercritical CO2 fluid is possible for transitional d-elements.





Similar content being viewed by others
REFERENCES
A. M. Vorobei, O. I. Pokrovskii, K. B. Ustinovich, et al., Sverkhkrit. Flyuidy: Teor. Prakt. 10 (2), 51 (2015).
E. Lemasson, S. Bertin, and C. West, J. Sep. Sci. 39, 212 (2016). https://doi.org/10.1002/jssc.201501062
P. B. Gomes, V. G. Mata, and A. E. Rodrigues, J. Supercrit. Fluids 41, 50 (2007). https://doi.org/10.1016/j.supflu.2006.08.018
J. W. King, Ann. Rev. Food Sci. Technol. 5, 215 (2014). https://doi.org/10.1146/annurev-food-030713-092447
A. Kaleva, S. Heinonen, J. P. Nikkanen, and E. Levänen, IOP Conf. Ser.: Mater. Sci. Eng. 175, 120 (2017). https://doi.org/10.1088/1757-899X/175/1/012034
E. P. da Silva, M. R. Guilherme, E. T. Tenório-Neto, et al., Mater. Lett. 136, 133 (2015). https://doi.org/10.1016/j.matlet.2014.07.156
C. Zhu, Y. Zhou, S. Fu, et al., ECS Trans. 69, 631 (2015). https://doi.org/10.1149/06917.0631ecst
D. S. Kim, Y. H. Shin, and Y. W. Lee, Chem. Eng. Commun. 202, 78 (2015). https://doi.org/10.1080/00986445.2013.825611
A. D. C. Permana, A. Nugroho, K. Y. Chung, et al., Chem. Eng. J. 241, 216 (2014). https://doi.org/10.1016/j.cej.2013.12.029
G. M. Kuz’micheva, Tonk. Khim. Tekhnol. 10 (6), 5 (2015).
E. S. Alekseev, A. Y. Alentiev, A. S. Belova, et al., Russ. Chem. Rev. 89, 1337 (2020). https://doi.org/10.1070/RCR4932?locatt=label:RUSSIAN
I. A. Konovalov, B. N. Mavrin, N. A. Prokudina, et al., Russ. Chem. Bull. 65, 2795 (2016).
K. A. Smirnova, V. V. Fomichev, D. V. Drobot, et al., Tonk. Khim. Tekhnol. 10 (1), 76 (2015).
I. E. Sokolov, I. A. Konovalov, R. M. Zakalyukin, et al., MRS Commun. 8, 59 (2018). https://doi.org/10.1557/mrc.2018.3
G. Oskam, A. Nellore, R. L. Penn, et al., J. Phys. Chem. B 107, 1734 (2003). https://doi.org/10.1021/jp021237f
Park Jin-Koo, Myoung Jung-Jae, Kyong Jin-Burm, et al., Bull. Korean Chem. Soc. 24, 671 (2003). https://doi.org/10.5012/bkcs.2003.24.5.671
Y. Zhang, J. Yang, and Y.-X. Yu, J. Phys. Chem. B 109, 133575 (2005). https://doi.org/10.1021/jp045741r
A. R. Teymourtash, D. R. Khonakdar, and M. R. Raveshi, J. Supercrit. Fluids 74, 115 (2013). https://doi.org/10.1016/j.supflu.2012.12.010
A. E. Lebedev, A. M. Katalevich, and N. V. Menshutina, J. Supercrit. Fluids 106, 122 (2015). https://doi.org/10.1016/j.supflu.2015.06.010
D. Borjan, M. Gracnar, Z. Knez, et al., Processes 10, 2275 (2022). https://doi.org/10.3390/pr10112275
R. A. Pierotti, Chem. Rev. 76, 717 (1976). https://doi.org/10.1021/cr60304a002
J. Emsley, The Elements, 3rd ed. (Oxford Univ. Press, Oxford, 1998).
G. M. J. Barca, C. Bertoni, L. Carrington, et al., J. Chem. Phys. 152, 154102 (2020). https://doi.org/10.1063/5.0005188
C. Adamo, J. Chem. Phys. 110, 6158 (1999). https://doi.org/10.1063/1.478522
Funding
This work was supported by the Russian Foundation for Basic Research, grant no. 18-29-06013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golubev, D.V., Sigov, A.S., Kolobanov, A.I. et al. Mechanism for the Formation of Nanoscale Oxides in a Medium of Supercritical CO2 Fluid. Russ. J. Phys. Chem. 97, 1515–1521 (2023). https://doi.org/10.1134/S0036024423070117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024423070117