Abstract
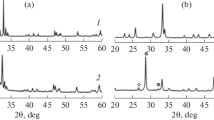
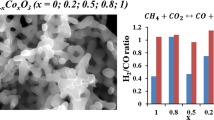
GdMn1 – xFexO3 (x = 0, 0.2, 0.5, 0.8, 1) perovskite oxides were prepared by a sol–gel method. The catalytic activity in dry reforming of methane was examined. XRD, BET, TPR, and TGA techniques have been used to characterize structural properties, reducibility and carbonization of the catalyst. The H2-TPR data show that an increase in the Mn content in the series of GdMn1 – xFexO3 compounds (x = 0, 0.2, 0.5, 0.8, 1) leads to a decrease in the reduction temperature. The study of the catalytic properties in the reaction of dry reforming of methane (DRM) showed that the catalytic activity of the studied compounds depends on the Fe content and increases in the series: GdMnO3 < GdMn0.8Fe0.2O3 < GdMn0.2Fe0.8O3 < GdFeO3.




Similar content being viewed by others
REFERENCES
B. Zhao, B. Yan, S. Yao, et al., J. Catal. 358, 168 (2018). https://doi.org/10.1016/j.jcat.2017.12.012
P. K. Yadav and T. Das, Int. J. Hydrogen Energy 44, 1659 (2019). https://doi.org/10.1016/j.ijhydene.2018.11.108
E. A. Filonova, A. N. Demina, and A. N. Petrov, Russ. J. Phys. Chem. A 81, 1591 (2007). https://doi.org/10.1134/S0036024407100081
Y. H. Wu, L. T. Luo, and W. Liu, Russ. J. Phys. Chem. A 84, 405 (2010). https://doi.org/10.1134/S0036024410030118
L. V. Yafarova, I. V. Chislova, I. A. Zvereva, et al., J. Sol-Gel Sci. Technol. 92, 264 (2019). https://doi.org/10.1007/s10971-019-05013-3
I. V. Chislova, A. A. Matveeva, A. V. Volkova, et al., J. Glass Phys. Chem. 37, 653 (2011). https://doi.org/10.1134/S1087659611060071
N. L. Ross, J. Zhao, and R. J. Angel, J. Solid State Chem. 177, 3768 (2004). https://doi.org/10.1016/j.jssc.2004.07.002
J. A. C. Vasquez, D. A. L. Téllez, C. A. Collazos, and J. R. Rojas, J. Phys.: Conf. Ser. 687 (1) (2016). https://doi.org/10.1088/1742-6596/687/1/012087
G. J. McCarthy, P. V. Gallagher, and C. Sipe, Mater. Res. 8, 1277 (1973).
M. W. Lufaso and P. M. Woodward, Acta Crystallogr., B 60, 10 (2004). https://doi.org/10.1107/S0108768103026661
L. Kepinski and P. Kraszkiewicz, Colloids Surf., A 124, 742 (2020). https://doi.org/10.1016/j.colsurfa.2020.124742
S. Zhao, L. Wang, Y. Wang, et al., J. Phys. Chem. Solids 116, 43 (2018). https://doi.org/10.1016/j.jpcs.2017.12.057
D. Pakhare and J. Spivey, Chem. Soc. Rev. 43, 7813 (2014). https://doi.org/10.1039/c3cs60395d
T. F. Sheshko and Y. M. Serov, Russ. J. Phys. Chem. A 86, 283 (2012). https://doi.org/10.1134/S0036024412020264
S. Z. Roginskii, M. I. Yanovskii, and A. D. Berman, Fundamentals of the Application of Chromatography in Catalysis (Nauka, Moscow, 1972) [in Russian].
M. Shah, S. Das, A. K. Nayak, et al., Appl. Catal. A 556, 137 (2018). https://doi.org/10.1016/j.apcata.2018.01.007
C. Ruocco, B. de Caprariis, V. Palma, et al., J. CO2 Util. 30, 222 (2019). https://doi.org/10.1016/j.jcou.2019.02.009
H. R. Gurav, S. Dama, V. Samuel, et al., J. CO2 Util. 20, 357 (2017). https://doi.org/10.1016/j.jcou.2017.06.014
ACKNOWLEDGMENTS
The publication has been prepared with the support of the “RUDN University Program 5-100.” The research was performed at the Center of Thermal Analysis and Calorimetry, Research Centre for X‑ray Diffraction Studies and Center for Studies in Surface Science of Research Park of St. Petersburg State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
FUNDING
This work was supported by the scholarship and grant of the President of the Russian Federation (nos. SP-1164.2019.1 and MK-480.2020.3).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yafarova, L.V., Silyukov, O.I., Kryuchkova, T.A. et al. The Influence of Fe Substitution in GdFeO3 on Redox and Catalytic Properties. Russ. J. Phys. Chem. 94, 2679–2684 (2020). https://doi.org/10.1134/S0036024420130324
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420130324