Abstract
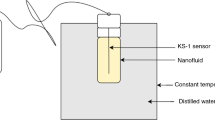
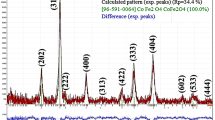
The acoustical and thermophysical properties of nanofluids is of great importance in heat exchange systems. Thus, these properties of nickel oxide (NiO) nanoparticles dispersed in ethylene glycol (EG) were studied after synthesizing and characterization of NiO nanoparticles. Speed of sound, acoustic impedance and free volume for NiO-EG nanofluids first increased upto 0.6 wt % and then decreased. However, viscosity, adiabatic compressibility and intermolecular free length first decreased upto 0.6 wt % and then increased. The density was observed to increase with increase in concentration and decrease with temperature. Speed of sound, viscosity and acoustic impedance decreases with increase in temperature, whereas, adiabatic compressibility, intermolecular free length and free volume increases. Thermal conductivity of NiO-EG nanofluids increased upto 0.6 wt % followed by its decrease, and it also decreased with the temperature at all concentrations. In conclusion, NiO-EG nanofluids upto 0.6 wt % concentration are very useful for nanofluids applications in thermal systems.





Similar content being viewed by others
REFERENCES
C. K. Mangrulkar and V. M. Kriplani, Int. J. Eng. Technol. 3, 136 (2013).
S. Aberoumand, H. Aberoumand, and K. Javaherdeh, Am. J. Adv. Sci. Res. 1, 375 (2013).
H. Xie, J. Wang, T. Xi, Y. Liu, and F. Ai, J. Appl. Phys. 91, 4568 (2002).
WanMeher, R. R. Yadav, K. L. Yadav, and S. B. Yadaw, Exp. Therm. Fluid Sci. 41, 158 (2012).
J. Hemalatha, T. Prabhakaran, and R. P. Nalini, Microfluid. Nanofluid. 10, 263 (2011).
N. T. Nguyen, A. Beyzavi, K. M. Ng, and X. Huang, Microfluid. Nanofluid. 3, 571 (2007).
C. He, T. Sasaki, Y. Shimizu, and N. Koshizaki, Appl. Surf. Sci. 254, 2196 (2008).
R. P. Singh, V. K. Shukla, R. S. Yadav, P. K. Sharma, P. K. Singh, and A. C. Pandey, Adv. Mater. Lett. 2, 313 (2011).
S. Kamila and J. K. Dash, J. Mol. Liq. 172, 71 (2012).
S. Kamila, S. K. Kamilla, and B. B. Swain, Phys. Chem. Liq. 45, 323 (2007).
T. L. Lai, Y. Y. Hong, G. Y. Gau, Y. Y. Shu, and C. B. Wang, Appl. Catal. B 68, 147 (2006).
Y. Nuli, S. Zhao, and Q. Qin, J. Power Sources 114, 113 (2003).
V. Biji and M. A. Khadar, Mater. Sci. Eng. A 304–306, 814 (2001).
R. H. Kodama, J. Magn. Magn. Mater. 200, 359 (1999).
J. S. Rowlinson, F. L. Swinton, J. E. Baldwin, A. D. Buckingham, and S. Danishefsky, Liquids and Liquid Mixtures (Butterworths, London, UK, 1982).
M. J. W. Povey, Ultrasonic Techniques for Fluids Characterization (USA, 1997).
B. Jacobson, Acta Chem. Scand. 5, 1214 (1951).
J. H. Hildebrand, J. Chem. Phys. 31, 1423 (1959).
L. Prigogine and V. Malhot, The Molecular Theory of the Solution (North-Holland, New York, 1957).
C. V. Suryanarayana and J. Kuppusamy, J. Acoust. Soc. Ind. 4, 75 (1976).
R. B. Bird, W. E. Stewart, and E. N. Lightfoot, Transport Phenomena (Wiley, New York, 2011).
M. N. Rashin and J. Hemalatha, J. Mol. Liq. 197, 257 (2014).
K. Karthik, G. K. Selvan, M. Kanagaraj, S. Arumugam, and N. V. Jaya, J. Alloys Compd. 509, 181 (2011).
M. Kanthimathi, A. Dhathathreyan, and B. V. Nair, Mater. Lett. 58, 2914 (2004).
M. Salavati-Niasari, N. Mir, and F. Davar, Polyhedron 28, 1111 (2009).
R. Kiruba, M. Gopalakrishnan, T. Mahalingam, A. Jeevaraj, and A. K. Solomon, J. Nanofluids 1, 97 (2012).
Y. Tan, Y. Song, and Q. Zheng, Nanoscale 4, 6997 (2012).
K. S. Suganthi, V. L. Vinodhan, and K. S. Rajan, Appl. Energy 135, 548 (2014).
M. N. Rashin and J. Hemalatha, Advanced Nanomaterials and Nanotechnology (Berlin, Heidelberg, 2013).
V. Gupta, A. K. Sharma, and M. Sharma, Chem. Sci. Trans. 3, 736 (2014).
S. Kamila and V. R. V. Gopal, Heliyon 5, e02445 (2019).
M. K. Praharaj, P. Mishra, S. Mishra, and A. Satapathy, Adv. Appl. Sci. Res. 3, 1518 (2012).
S. Panda and A. P. Mahapatra, J. Chem. Pharmaceut. Res. 6, 818 (2014).
R. R. Naik, S. V. Bawankar, and V. M. Ghodki, J. Polym. Biopolym. Phys. Chem. 3, 1 (2015).
M. Umadevi and R. Kesavasamy, Int. J. Res. Chem. Environ. 2, 157 (2012).
B. Tajik, A. Abbassi, M. Saffar-Avval, and M. A. Najafabadi, Powder Technol. 217, 171 (2012).
R. Kiruba and A. K. S. Jeevaraj, Integr. Ferroelectr. 150, 59 (2014).
M. Kumar, N. Sawhney, A. K. Sharma, and M. Sharma, Ind. J. Pure Appl. Phys. 55, 574 (2017).
Y. Xuan and Q. Li, Int. J. Heat Fluid Flow 21, 58 (2000).
ACKNOWLEDGMENTS
We are thankful to Department of Chemistry (University of Jammu) for providing essential facilities and support for conducting present study. We also gratefully acknowledge SAIF STIC (Kochi), SAIF Chandigarh, CIL Chandigarh for providing the facilities for characterization studies. The first author is highly thankful to CSIR- UGC, New Delhi, India for granting Junior Research Fellowship (JRF) during this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gupta, V., Magotra, U., Sharma, A.K. et al. Investigations on Acoustical and Thermal Properties of Ethylene Glycol Based Nickel Oxide Nanofluids: Concentration and Temperature. Russ. J. Phys. Chem. 94, 2312–2318 (2020). https://doi.org/10.1134/S0036024420110096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420110096