Abstract
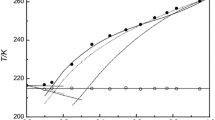
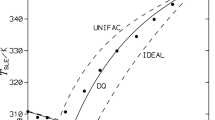
Liquid-solid phase equilibria are studied in the eutectic benzoic acid-naphthalene system by means of thermic analysis (DTA, CTA), on the basis of which the liquidus line and eutectic point (x e ≈ 50 mol %, T e ± 340 K) are determined and the phase diagram is constructed. Average precrystallization supercooling temperatures ΔT −L of the liquid phase relative to liquidus temperature T L are determined, allowing us to locate the region of solution metastability on the phase diagram. Excessive functions of the components in the liquid phase are found via thermodynamic modeling using the Margules equation and experimental data. The boundaries of the region of liquid solution metastability are estimated from the thermodynamic conditions of solution stability.
Similar content being viewed by others
References
U. S. Raiand and K. D. Mandal, Can. J. Chem. 67, 239 (1989).
B. L. Sharma, Mater. Sci. Eng. 25, 11 (1994).
R. N. Rai and R. S. B. Reddi, Thermochim. Acta 496, 13 (2009).
K. P. Sharma, P. R. Shakya, and R. Rai, Sci. World 10(10), 91 (2012).
V. D. Aleksandrov, V. A. Postnikov, and N. V. Shchebetovskaya, Russ. J. Phys. Chem. A 84, 906 (2010).
V. D. Aleksandrov and N. V. Shchebetovskaya, Ukr. Khim. Zh. 78(3), 32 (2012).
V. D. Aleksandrov and N. V. Shchebetovskaya, Materialovedenie, No. 7, 13 (2012).
C. H. Sorum and E. A. Durand, J. Am. Chem. Soc. 74, 1071 (1952).
W. Wendlandt, Thermal Analysis Methods (Wiley, New York, 1997; Mir, Moscow, 1978).
S. D. Sharma and K. Sagara, Int. J. Green Energy, No. 2, 1 (2005).
V. A. Rabinovich and Z. Ya. Khavin, Concise Chemical Handbook (Khimiya, Leningrad, 1991) [in Russian].
S. Walas, Phase Equilibria in Chemical Technology (Butterworth-Heinemann, Boston, London, Oxford, UK, 1985; Mir, Moscow, 1989), part 1.
N. A. Gokcen, J. Phase Equilib. 17, 50 (1996).
V. M. Glazov and L. M. Pavlova, Chemical Thermodynamics and Phase Equilibria (Metallurgiya, Moscow, 1981) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.A. Postnikov, 2014, published in Zhurnal Fizicheskoi Khimii, 2014, Vol. 88, Nos. 7–8, pp. 1153–1157.
Rights and permissions
About this article
Cite this article
Postnikov, V.A. Phase diagram of the eutectic benzoic acid-naphthalene system in the temperature range of 300–400 K. Russ. J. Phys. Chem. 88, 1336–1339 (2014). https://doi.org/10.1134/S0036024414080238
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024414080238